Revisión sistemática
← vista completaPublicado el 13 de mayo de 2021 | http://doi.org/10.5867/medwave.2021.04.8190
Oxígeno con cánula de alto flujo para el tratamiento de la bronquiolitis aguda del lactante: revisión sistemática y metanálisis
High-flow oxygen nasal cannula for treating acute bronchiolitis in infants: A systematic review and meta-analysis
Abstract
Introduction Oxygen therapy through a high-flow nasal cannula is thought to improve the work of breathing and the comfort of patients with acute bronchiolitis. It is widely used in hospital wards and critical care of pediatric patients. However, there is uncertainty on the magnitude of the effect on critical and important outcomes in these patients.
Objectives The objective of this review is to evaluate the available evidence on the use of oxygen administered through high-flow cannula versus low-flow oxygen for the treatment of acute bronchiolitis in children under two years of age.
Methodology We carried out a systematic review and a meta-analysis following the PRISMA standards for reporting. The search was carried out in electronic databases by two researchers independently. The evidence was summarized using the GRADE methodology.
Results Six randomized and non-randomized clinical trials were included, including 1867 individuals younger than 24 months of age with acute bronchiolitis in pediatric emergency, hospitalization, and intensive care services. Mortality was not reported in the included studies. Treatment failure occurred in 108/933 in the high flow group and 233/934 in the low flow group (relative risk: 0.46; 95% confidence interval: 0.35 to 0.62), which shows 11.7% less treatment failure (95% confidence interval between 7.9% and 14.5% less) in the high flow group with a number needed to treat of 7.5 (95% confidence interval 6 to 10) with moderate certainty in the evidence.
Conclusion The use of humidified and heated oxygen with high flow compared to oxygen at low flow is probably associated with decreased treatment failure in children younger than two years with acute bronchiolitis. There is uncertainty about the effect on hospitalization days and clinical progression.
Main messages
|
Introduction
Bronchiolitis is the most common lower respiratory tract infection in infants and represents an important cause of hospitalization in this age group. It is estimated that 11 to 12% of infants are affected in the first year of life, and 1 to 2% of them require hospitalization [1]. Of those admitted to emergency rooms and hospitalization, 10% of previously healthy children and 36% of those with comorbidities will require admission to a pediatric intensive care unit. This group of children has an associated mortality of 1% [2],[3].
Treatment by traditional low-flow oxygen therapy with a mask or nasal cannula has limitations due to the application of dry oxygen and the impossibility of using high flow [4],[5].
When this therapeutic approach is ineffective, noninvasive ventilation strategies are used, mainly through continuous positive airway pressure.
The application of noninvasive ventilation presents difficulties in small infants, especially those weighing less than 7 kg. In addition to the technical limitations related to the interface used, the flows generated, and the equipment available, continuous positive airway pressure/noninvasive ventilation can be challenging, requires highly trained nursing and medical staff, is not always tolerated by patients and, therefore, in the past, has been restricted to the intensive care unit [6],[7].
Heated humidified high-flow nasal cannula oxygen therapy provides heated and humidified gas at high flow rates to improve breathing and patient comfort. The addition of heat and high levels of humidification allows high flows of air and oxygen, achieving concentrations close to 100%. This requires systems with appropriate oxygen mixers and flowmeters. These high-flow oxygenation systems are convenient, simple, and have the advantage of not interfering with feeding [8],[9].
In pediatric wards, high-flow oxygen therapy through a nasal cannula has become a widely used new method to provide respiratory support for respiratory diseases in infants and newborns. Clinical studies in infants with acute bronchiolitis show benefits in some surrogate outcomes (respiratory frequency, days of hospitalization, among others) [10].
We set out to conduct a systematic review to synthesize the available evidence on the use of high-flow nasal cannula oxygen compared to low-flow oxygen for the treatment of acute bronchiolitis in children under two years of age.
Methods
A multidisciplinary team, free of conflicts of interest, was formed to conduct a systematic review of the use of high-flow cannulae versus oxygen administered through low-flow systems to treat acute bronchiolitis in the population of children under two years of age.
This study was conducted following PRISMA (Preferred Reported Items in Systematic Reviews and Meta-analysis) guidelines for the conduct and publication of systematic reviews [11].
The protocol was previously published in the PROSPERO International Prospective Register of Systematic Reviews. Registration number: CRD42020169993 [12].
Research question
Should heated humidified high-flow nasal cannula be used versus conventional low-flow oxygen therapy to treat acute bronchiolitis in children under two years of age?
PICO question
P: patients younger than 24 months with a presumptive diagnosis of acute bronchiolitis.
I: nasal cannula with high-flow oxygen.
C: low-flow oxygen.
O: mortality, treatment failure (defined as the need for noninvasive ventilation or intubation), severity score, length of hospitalization, treatment-related adverse events.
Data sources
Two reviewers independently conducted a sensitive search in the following bibliographic databases: PubMed, EMBASE, LILACS, Cochrane Library, Epistemonikos, and Tripdatabase. A search was also made of other sources and repositories of health technology assessment agencies and national and international agencies. An evidence matrix was performed to identify among the primary studies that made up the different systematic reviews initially selected, works that had not emerged from other search strategies. Additional searches of the references were also carried out through Crossref.
This is the example search strategy in Medline/PubMed, which included the following terms:
#1 MeSH descriptor Oxygen Inhalation Therapy explode all trees #2 intubation rates*.
#3 (#1 AND #2) #4 ((high flow (nasal or prong or cannula)) or (nasal near oxygen)):ti,ab #5 (#3 OR #4).
No language restriction was performed. In addition, to identify additional literature, Google Scholar was searched, and experts were contacted. These searches were conducted according to the platform type, and free language terms were used in the search. The searches were conducted until July 1, 2020.
More details about the complete search can be found in Annex 1.
Selection of studies
We searched for randomized controlled trials, non-randomized, prospective, and analytical studies that included a control group in children under 24 months of age with a presumptive diagnosis of acute bronchiolitis. The intervention was high-flow oxygen nasal cannula, and the comparator was some form of low-flow oxygen administration.
The following outcomes were considered in order of clinical relevance: death (critical, 9), treatment failure, defined as the need for mechanical ventilation or noninvasive ventilation (mechanical ventilation or noninvasive ventilation) (critical, 7), days of hospitalization (important, 6), severity scores (important, 4), adverse events (not important, 3).
Data collection
Studies were selected by two reviewers independently based on inclusion and exclusion criteria. Differences were defined by agreement between the reviewers. References of identified systematic reviews were also reviewed. Two reviewers independently extracted the evidence of the selected outcomes in electronic spreadsheets designed for this purpose.
Risk of bias
Risk of bias assessment was performed through the risk of bias tool suggested by Cochrane (RoB-2) [13]. Two operators independently assessed the risk of bias in the trials (Annex 2 and Figure 2). For each trial and outcome, the reviewers rated them as follows:
- Low risk of bias.
- Some concerns, probably low risk of bias.
- Some concerns, probably high risk of bias.
- High risk of bias.
Risk of bias domains: Bias arising from the randomization process; bias due to deviations from the intended intervention; bias due to missing outcome data; bias in outcome measurement; bias in the selection of outcome reports, including deviations from the recorded protocol; and bias arising from early termination in benefit.
Differences between operators were defined by a third party where necessary.
Measures of effect and statistical analysis
Analysis of the results was performed using relative risk for binary outcomes and mean difference for continuous outcomes, together with their 95% confidence interval. The Mantel and Haenszel random-effects model was used for meta-analysis.
R (RStudio Version 1.3.1073 © 2009-2020 RStudio, PBC) was used for meta-analytic calculations, risk of bias, publication bias, and the production of traffic light, summary, forest plot, and funnel plots.
Certainty of evidence
The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess the certainty in the body of evidence for each comparison. The GRADE tool was used to create the summary tables of findings, and the information on the studies and outcomes included was extracted in spreadsheets. Subsequently, the selected studies were synthesized, and the evidence profiles were constructed with the help of the GRADE website [14],[15].
We rated the certainty for each comparison and outcome as high, moderate, low, low, or very low, based on the risk assessment domains of bias, inconsistency, indirect evidence, publication bias, and imprecision.
The GRADE evidence profiles are available in Table 2 and Annex 2.
Analysis of sample power and optimal intervention size
To detect appropriate sample power, calculation of the optimal intervention size was performed, using R (RStudio Version 1.3.1073 © 2009-2020 RStudio, PBC), assuming an effect d (the hypothetical or plausible overall effect size of an intervention in the study compared with control) of SMD 0.15, a number k of studies of 5, the number of patients in intervention and control branches of 200 (n1 and n2) and an α level of 0.05 and moderate heterogeneity, with a power of 81.75%.
Subgroup analysis and publication bias
Subgroup analyses were performed according to the risk of bias of the included studies (low versus high risk of bias: LOW_ROB versus HIGH_ROB). The presence of publication bias was analyzed through the performance of a funnel plot and the Eggers test statistic [16].
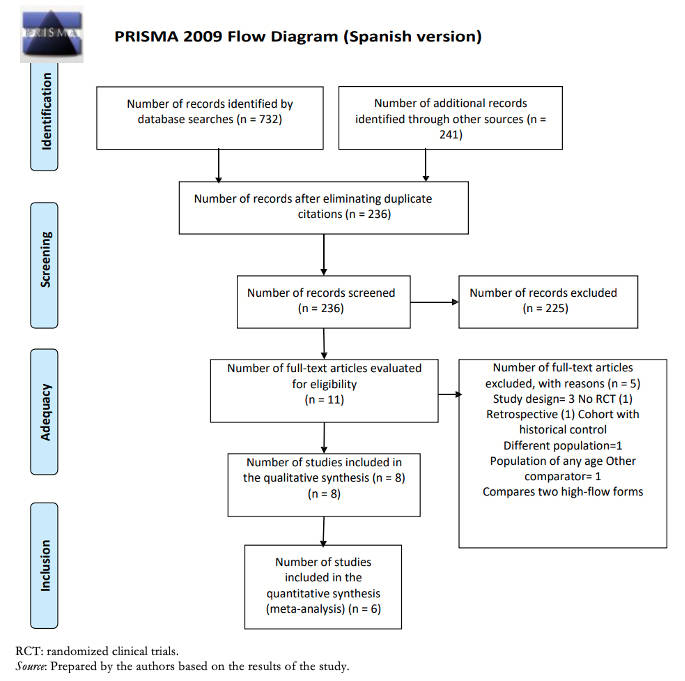 Full size
Full size Participants were in all cases under 24 months of age, while two studies only included children under 12 months of age and in another study only children under six months of age. The clinical setting was in all cases the inpatient, emergency, and pediatric critical care area. The intervention used was a nasal cannula with humidified and heated oxygen at high flow with a flow rate of 1 to 2 L/kg/min, with a maximum in all cases of 20 L. The comparator was low-flow oxygen with nasal cannula or a mask with 100% oxygen. A summary of the studies included in this review [17],[18],[19],[20],[21],[22] can be found in Table 1.
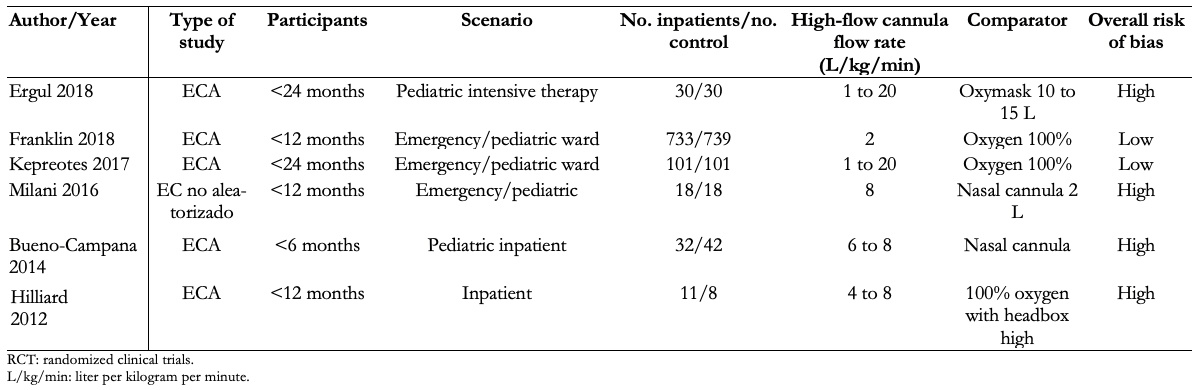 Full size
Full size The excluded studies and the reasons for their exclusion [23],[24],[25],[26],[27],[28],[29] are described in Annex 2.
The overall risk of bias of the included studies was high in four of them, due to problems in blinding of participants and reporting of included outcomes, whereas, in two, Franklin et al. 2018 [19] and Kepreotes et al. 2017 [17], it was low (Figure 2).
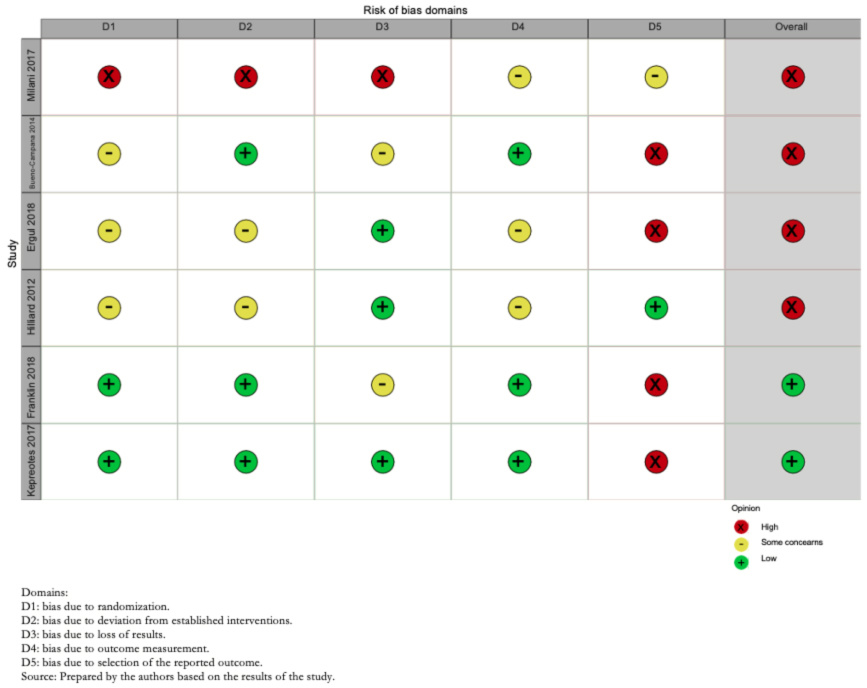 Full size
Full size Death outcome
Most of the studies included in this review did not report the outcome of death. One of them, Kepreotes et al. 2017 [17], reported that there were no deaths during the follow-up period.
Treatment failure outcome
Treatment failure (need for noninvasive, invasive ventilation, or orotracheal intubation) occurred in 108/933 in the humidified high-flow nasal cannula group and 233/934 in the low-flow group (relative risk: 0.46; 95% confidence interval: 0.35 to 0.62). This means in absolute numbers 11.7% less treatment failure (95% confidence interval between 7.9% and 14.5% less) in the high-flow humidified nasal cannula group (Table 2).
The certainty in the evidence regarding this outcome was moderate due to the high risk of bias in the included studies (Figure 3).
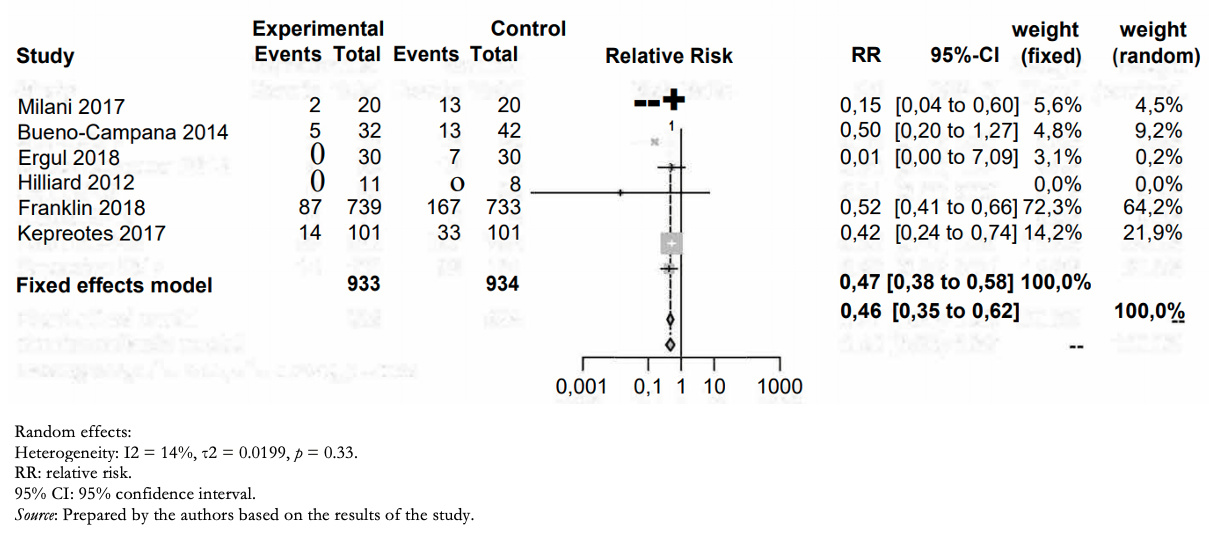 Full size
Full size Outcome days of hospitalization
Patients treated with humidified high-flow nasal cannula versus those treated with low-flow had a mean difference in inpatient days of −0.55 (between 1.92 days less and 0.85 days more) (Figure 4).
The certainty of the evidence for this outcome was very low due to imprecision (wide confidence intervals including risks and benefits), heterogeneity (high I2, overlapping confidence intervals), and risk of bias in the included studies.
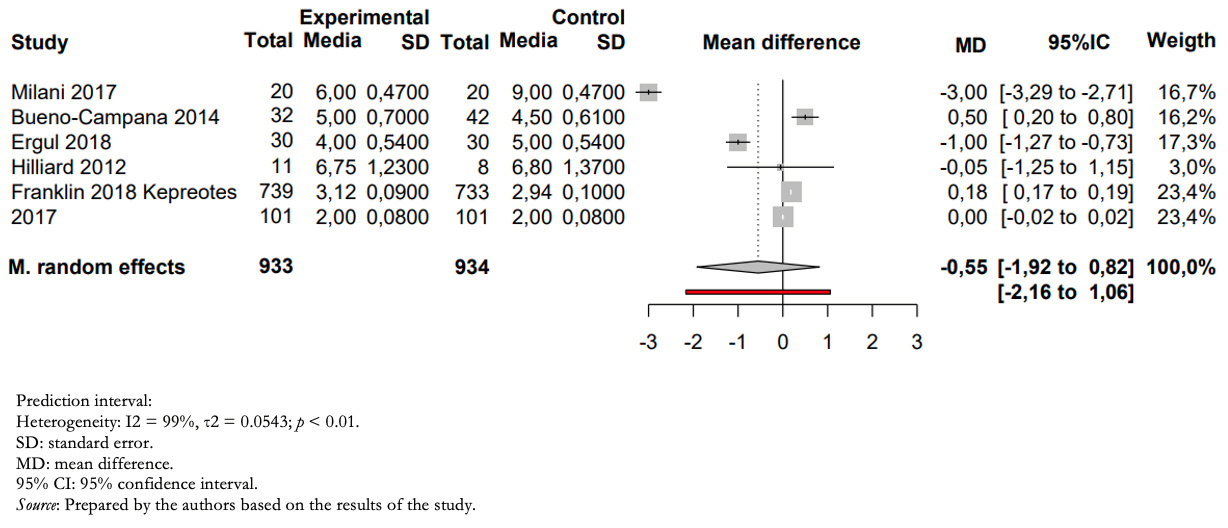 Full size
Full size Disentanglement severity scores
The severity scores used varied widely among studies. While Hilliard et al. [18] reported no change in severity scores, Franklin et al. [19], Ergul et al. [21], and Milani et al. [22] used clinical parameters such as respiratory rate and effort, and feeding ability, which were then reported as individual outcomes in response to treatment.
Kepreotes and colleagues [17], on the other hand, initially assessed children through the Health Initial Severity Assessment of Bronchiolitis score, which also includes different clinical parameters such as cyanosis and oxygen saturation, using these parameters to assess treatment failure and time to treatment failure.
Finally, Bueno Campaña et al. [20] used the Respiratory Distress Assessment Instrument (RDAI), which measures the presence of wheezing, crackles, and rib retraction. The change in this score before and after treatment was used to measure the efficacy of the assigned treatment. In this study, no difference was found between the means of the two groups (p = 0.24): mean difference −1.22 (95% confidence interval: 2.99 to 0.55).
Treatment-related adverse events outcome
The included studies reported no intervention- or comparator-related adverse events.
Other analyses
For the outcome of treatment failure, heterogeneity was low among the included studies, and the direction and size of the effect were maintained after subgroup analysis between low and high risk of bias (Annex).
Regarding the outcome days of hospitalization, heterogeneity between studies was high I2 = 99% and was maintained after sensitivity analysis between studies with high and low risk of bias (Annex 2).
The publication bias analysis shows asymmetry in the funnel plots (Annex 2, Figures 3 and 4) and Eggers test at the limit of statistical significance intercept: −1.31 (95% confidence interval: −2.27 to −0.45), p = 0.06. However, the number of studies is less than 10, not enough to suspect publication bias.
Discussion
The absolute and relative estimates of effect and the significance of the outcomes can be seen in Table 2.
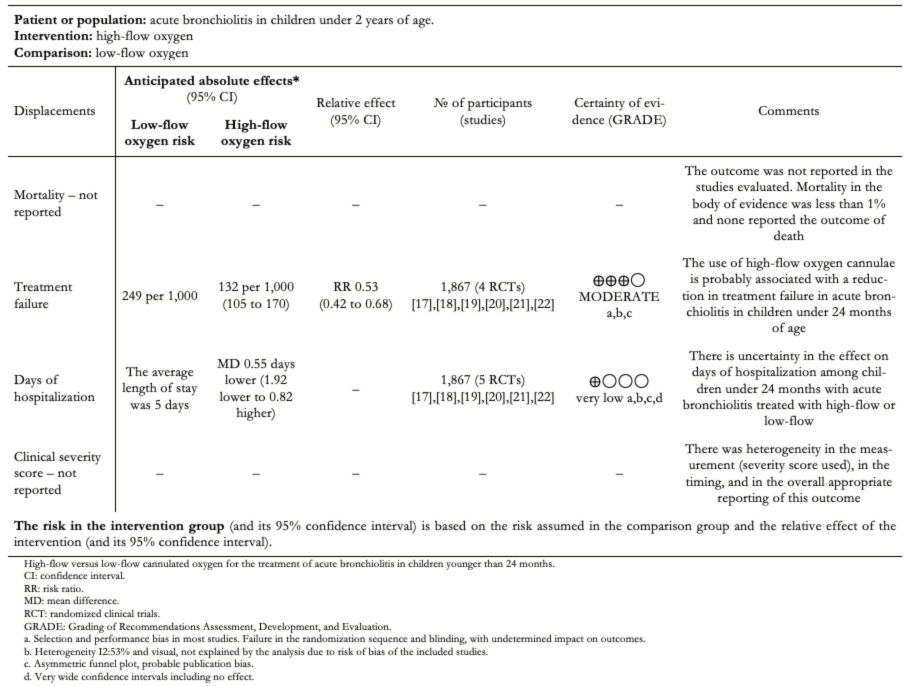 Full size
Full size This meta-analysis was conducted with high-quality standards, following regulations for conducting and reporting systematic reviews, together with PRISMA meta-analysis. We included the best available evidence through an exhaustive review of the literature and assessment of the risk of bias within the included studies and the publication bias among the studies. In addition, the evidence summary was performed using the GRADE methodology to determine the effect estimates and the degree of certainty of the body of literature included in the review.
The evidence analyzed shows that the use of oxygen through humidified and heated high-flow cannula is probably associated with an absolute reduction of 11.7% in treatment failure in infants with acute bronchiolitis under 24 months of age, with 7.5 patients needing to be treated with the intervention to reduce one event (number needed to treat 7.5; 95% confidence interval: 6 to 10).
Regarding days of hospitalization, there is uncertainty in the magnitude of the effect of humidified high-flow nasal cannula compared to standard treatment, so high-flow oxygen therapy may not be associated with a change in days of hospitalization. This is supported by low certainty of the evidence due to imprecision and risk of bias in the included studies.
We were unable to properly assess mortality since, in the studies reviewed, mortality remained below 1%. In the follow-up cohorts of patients with acute bronchiolitis, mortality was not reported; therefore, there is no evidence available on the effect of high-flow oxygen on death in the treatment of acute bronchiolitis in this age group.
However, severity scores were not included because they could not be adequately assessed, given that there is significant imprecision in the timing of their use, in the types of scores assessed, and in their proper reporting.
No intervention- or control-related adverse events were reported.
Conclusions
The use of high-flow humidified and heated oxygen compared with low-flow oxygen is likely associated with decreased treatment failure in children under two years of age with acute bronchiolitis.
There is uncertainty in the magnitude of the effect on hospital days and clinical progression scores.
The available evidence is insufficient to assess the effect of the intervention on mortality in the population evaluated.
Notes
Authorship contributions
FT: conceptualization, methodology, data validation, formal analysis, preparation and writing, editing and supervision. IA: conceptualization, methodology, formal analysis and supervision. GV: conceptualization, methodology, data validation and editing. VS: methodology and supervision. PH: formal analysis, preparation and writing, and editing. GC: preparation and writing and editing.
Competing interests
The authors completed the ICMJE conflict of interest statement and declared that they received no funding for the completion of this article; have no financial relationships with organizations that may have an interest in the published article in the last three years; and have no other relationships or activities that may influence the publication of the article. Forms can be requested by contacting the responsible author or the Editorial Board of the Journal.
Funding
The authors declare that they had no external sources of financing for the present study.
Ethics
The present study did not require evaluation by an ethics committee since it is a systematic review and uses secondary data sources.
Annex with supplementary material
Supplementary material, search strategy, tables, and graphs not present in this manuscript are available at the following link.
Registration of the study protocol
PROSPERO, CRD42020169993.
Language of submission
Spanish.

