Epistemonikos summaries
← vista completaPublished on April 28, 2015 | http://doi.org/10.5867/medwave.2015.03.6129
Is there a role for digitalis in chronic heart failure?
¿Tienen un rol los digitálicos en la insuficiencia cardíaca crónica?
Abstract
The main clinical guidelines recommend the use of digitalis for chronic heart failure when moderate to severe symptoms persist after standard therapy, even though there is controversy about its efficacy and security. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including 13 randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded the use of digitalis for chronic heart failure probably leads to little or no decrease in mortality, but might reduce hospitalizations and clinical deterioration. However, the certainty of the evidence is low.
Problem
Digitalis have been in use for treatment of heart failure for more than two centuries. However, their effects on heart failure are controversial. On one hand, they would improve symptoms and exercise tolerance. On the other hand, they might increase mortality, especially when there is underlying ischemic heart disease. Furthermore, they carry a high risk of adverse effects.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence |
We found three systematic reviews [1],[2],[3] including 13 randomized controlled trials that are reported in 15 articles [4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18]. |
|
What types of patients were included |
All studies considered heart failure of any etiology, being the most frequent ischemic. Only five studies included patients with reduced ejection fraction: < 45% [7],[18], < 40% [14] and < 35% [11],[16]. |
|
What types of interventions were included |
All studies evaluated digoxin, eight using dose adjustments to reach a specific serum level [8],[9],[10],[11],[13],[14],[16],[17]. |
|
What types of outcomes were measured |
Total mortality or heart failure mortality; hospitalization for any cause or for heart failure, emergency room visits, clinical deterioration, quality of life, walking test, neurohumoral markers and echocardiographic parameters. |
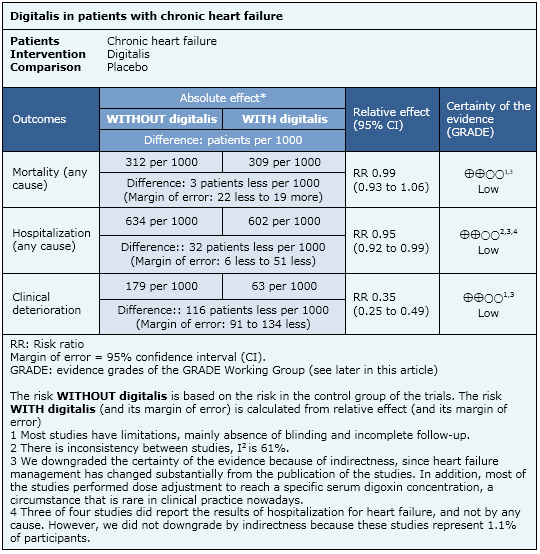
Summary of findings
The following information is based on 13 randomized trials that included 8,304 patients. Only eight studies reported total mortality. One study reported hospitalization by any cause, four reported hospitalization from heart failure and 12 reported clinical deterioration.
- Use of digitalis in chronic heart failure might lead to little o no decrease in mortality. The certainty of the evidence is low.
- Digitalis might lead to a reduction in hospitalization and clinical deterioration, but the certainty of the evidence is low.
Digitalis for chronic heart failure
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence
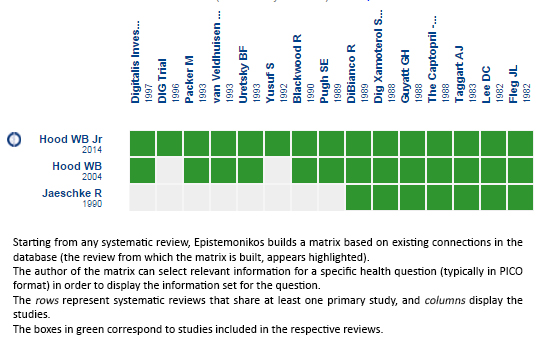 Full size
Full size Follow the link to access the interactive version Digitalis for chronic heart failure

Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

