Epistemonikos summaries
← vista completaPublished on June 12, 2015 | http://doi.org/10.5867/medwave.2015.6160
Should we add vancomycin antibiotic powder to prevent post operative infection in spine surgery?
¿Debemos agregar vancomicina en polvo a la profilaxis antibiótica en cirugía de columna?
Abstract
Intravenous antibiotic prophylaxis is routinely administered to prevent surgical site infection in spinal surgery. Adding intrawound vancomycin powder before surgical closure might further decrease infection risk. However, its use is controversial. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified six systematic reviews that considered 16 studies, including one randomized controlled trial. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded vancomycin probably does not decrease the risk of infection in low risk surgery, but there is uncertainty about its effects in populations or surgeries with a higher risk because the certainty of the evidence is very low.
Problem
The infection rate after spinal surgery ranges from 0.5 to 12 %. For decades, efforts have been made in order to implement different measures to reduce this risk and then to improve surgical outcomes. Adding intrawound vancomycin powder could decrease the risk of infection and associated complications.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1-6] that consider 16 primary studies[7-22], including only one randomized controlled trial [21]. |
|
What types of patients were included |
The 16 studies included adults; three studies included posterior cervical surgery [7],[15],[19], six studies (including the only randomized trial) cervical and posterior thoracolumbar surgery [8],[11],[14],[17],[20],[21], four posterior thoracolumbar surgery [12],[13],[18],[22], one posterior lumbar [16] and two studies did not specify the type of surgery [9],[10]. Three studies (including the only randomized trial) analyzed separately instrumented and non-instrumented surgery [10],[16],[21]. |
|
What types of interventions were included |
The intervention was vancomycin powder. Nine studies (including the randomized) administered one gram of vancomycin powder [7-9],[14],[16],[19-22], three studies used two grams [12],[13],[18], two studies 0.5 to two grams [10,11], one study one to two grams [17] and one study 500 mg [15]. All studies compared against standard treatment which corresponds to intravenous cefazolin. |
|
What types of outcomes were measured |
Risk of infection, Staphylococcus aureus infection, pseudarthrosis. |
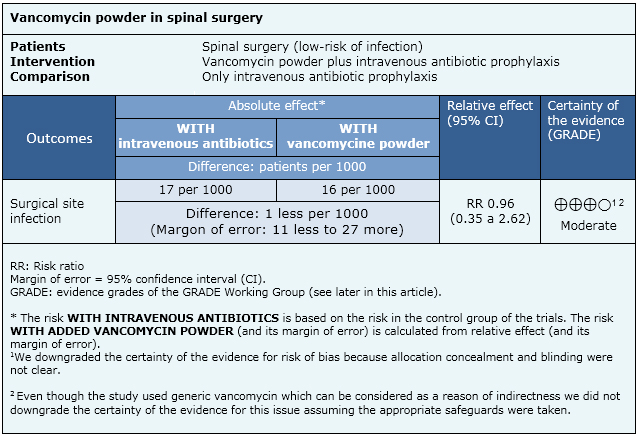
Summary of findings
The information on the effects of vancomycin powder on the surgical site is based on one randomised controlled trial including 907 patients. We conducted an evaluation of the certainty of the evidence coming from 15 non-randomised studies, which produced lower certainty tan the only randomised trial. So, we considered it for the formulation of key messages and considerations for decision-making, but not for the summary of findings table.
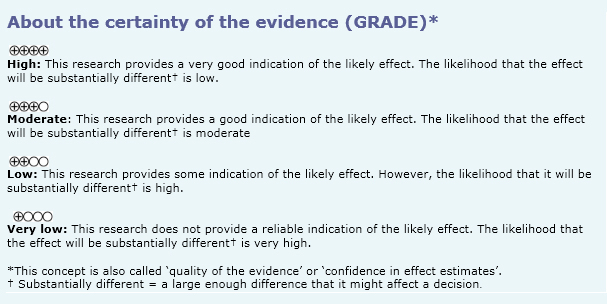
- Vancomycin powder probably does not decrease surgical site infection in low-risk spinal surgery. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
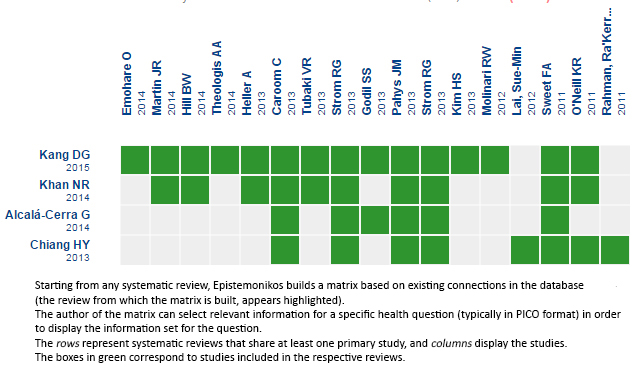 Full size
Full size Follow the link to access the interactive version Vancomycin powder vs endovenous antibiotic prophylaxis to avoid surgical site infection in patients with spine surgery
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

