Epistemonikos summaries
← vista completaPublished on July 9, 2015 | http://doi.org/10.5867/medwave.2015.6179
What are the effects of adding azathioprine to corticosteroids in polymyositis?
¿Sirve agregar azatioprina a los corticoides en pacientes con polimiositis?
Abstract
The treatment of polymyositis is based on corticosteroid therapy, with addition of azathioprine for non responsive cases or as an attempt to diminish corticosteroids requirements. However, there is no clear evidence of its benefit in controlling symptoms. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified only one systematic review including one pertinent randomized trial. We generated a summary of findings following the GRADE approach. We concluded there is uncertainty if azathioprine improves or not muscular strength in polymyositis because the certainty of the evidence is very low.
Problem
Corticosteroids constitute the standard treatment for dermatomyositis and polymyositis. Given their multiple short and long term side effects there is interest in other immunomodulators, such as azathioprine, especially for non-responsive cases or as an attempt to diminish corticosteroids requirements. However, there is no clear evidence of its benefit in controlling symptoms, and it also carry important side effects.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found one systematic review [1] including only one pertinent randomized controlled trial [2]. |
|
What types of patients were included |
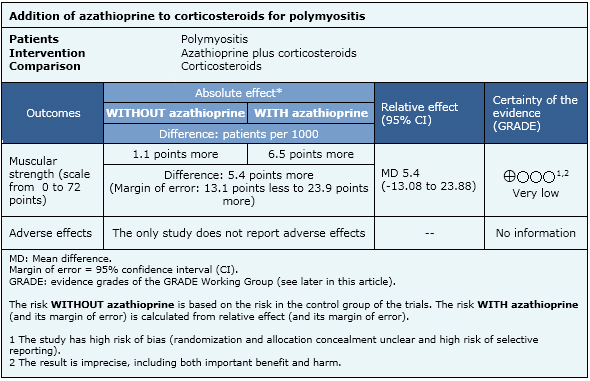
The study included adults with polymyositis diagnosed with Bohan and Peter criteria and positive muscle biopsy Patients with previous corticosteroid or immunosuppresive therapy were excluded. |
|
What types of interventions were included |
Azathioprine 2mg/kg qd plus prednisone 60 mg qd, compared with prednisone 60 mg qd. |
|
What types of outcomes were measured |
Improvement in muscular strength evaluated in each muscular group (score from 0= no deficit, to -140= maximum deficit), serum creatine kinase, muscular biopsy (0= normal, to 36= maximum inflammation). |
Summary of findings
The information on the effects of azathioprine is based on one randomized controlled trial [2] including 16 patients.
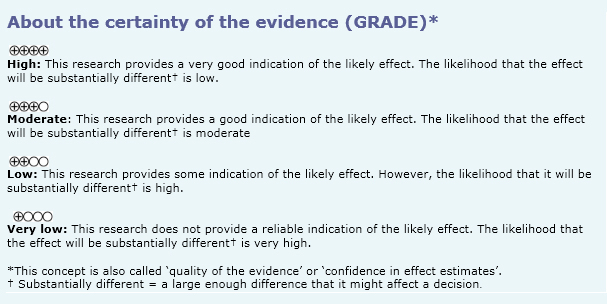
- There is uncertainty if azathioprine improves or not muscular strength in polymyositis. The certainty of the evidence is very low.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
 Full size
Full size Follow the link to access the interactive version Corticosteroids plus azathioprine versus corticosteroids alone for polymyositis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

