Resúmenes Epistemonikos
← vista completaPublicado el 14 de agosto de 2015 | http://doi.org/10.5867/medwave.2015.6209
¿Es efectivo el rituximab para inducir remisión en las vasculitis asociadas a ANCA?
Is rituximab effective for induction of remission in ANCA-associated vasculitis?
Abstract
Adding rituximab to the treatment with corticosteroids has been proposed as a therapeutic alternative for inducing remission in anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, especially when fertility is a concern, or when there is contraindication or intolerance to cyclophosphamide.
Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified only one systematic review including three pertinent randomized controlled trials. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded rituximab may slightly increase induction of remission rate, but it may also increase the risk of infection. It is not clear whether it increases the risk of cancer, or whether increases or decreases mortality because the certainty of the evidence is very low.
Problem
Immunosuppression with cyclophosphamide and corticosteroids has constituted the standard treatment for induction of remission in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis for years. Given the multiple adverse effects of cyclophosphamide, alternatives have been searched, such as the anti-CD20 antibody rituximab.
Clinical guidelines recommend it as a therapeutic alternative, especially in patients concerned about fertility preservation that maintain disease activity after standard treatment, or those that have contraindication or do not tolerate it.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found one systematic review [1] including 37 primary studies reported in 42 references Three studies correspond to randomized controlled trials, reported in eight references [2],[3],[11],[13],[20],[21],[37],[43]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
All studies included patients with ANCA-associated vasculitis. All studies included patients with Wegener’s granulomatosis or microscopic polyangiitis, and two studies also included renal limited vasculitis [2],[11],[37],[43]. Average age was 52 years [3],[13],[20],[21], 68 years [37],[43] and it was not reported in one study [2],[11]. Two studies reported the percentage of patients with new disease: 66% [3],[13],[20],[21] and 100% [37],[43]. The degree of disease activity measured with BVAS was 8.4 [3],[13],[19],[20],[21],[37],[43], and it was not reported in one study [2],[11]. |
|
What types of interventions were included |
All studies considered rituximab as the intervention. Two studies [3],[13],[20],[21],[37],[43] administered 375 mg/m2/week during four weeks. One study [2],[11] administered an infusion of 500 mg per day at days 1 and 15, then at 5.5 months and when completing 18 months. One study [37],[43] added cyclophosphamide 15 mg/kg during the first and third rituximab infusion. Two studies [3],[13],[20],[21],[37],[43] used cyclophosphamide as comparison. One of them [3],[13],[20],[21] employed a dose of 2 mg/kg/day and the other [37],[43] used 15 mg/kg every 2 weeks for the first three doses, then every 3 weeks until achieving remission. Both studies switched cyclophosphamide to azathioprine after achieving remission. The third study [2],[11] used azathioprine as comparison in an initial dose of 2 mg/kg/day during 22 months. |
|
What types of outcomes |
Induction and maintenance of remission, serious adverse events, defined as hospitalizations, cancer or mortality; other adverse effects as infections or hematological events. |
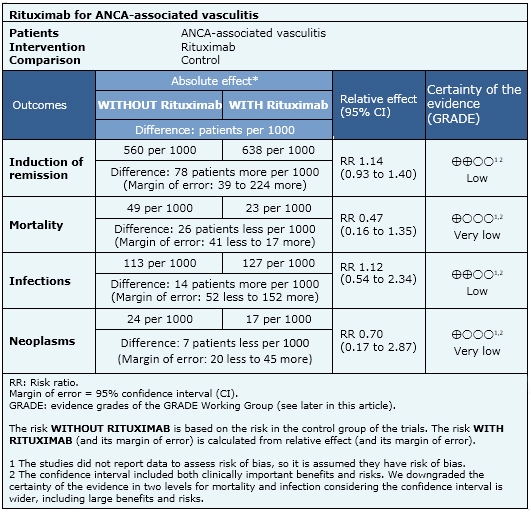
Summary of findings
The information on the effects of rituximab is based on three randomized trials including 350 patients. All studies reported mortality and adverse effects. Only two studies reported remission [3],[13],[20],[21],[37],[43].
- Rituximab may slightly increase induction of remission rate. The certainty of the evidence is low.
- Rituximab may increase the risk of infection. The certainty of the evidence is low.
- It is not clear whether rituximab increases the risk of cancer because the certainty of the evidence is very low.
- It is not clear whether rituximab increases or decreases mortality because the certainty of the evidence is very low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
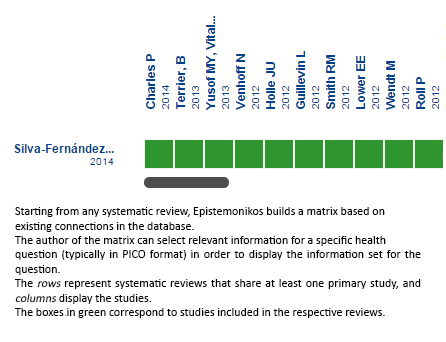 Full size
Full size Follow the link to access the interactive version: Rituximab in ANCA-associated renal vasculitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

