Resúmenes Epistemonikos
← vista completaPublicado el 18 de agosto de 2015 | http://doi.org/10.5867/medwave.2015.6218
¿Es el cierre percutáneo de la orejuela de la aurícula izquierda comparable al tratamiento anticoagulante en fibrilación auricular?
Is percutaneous closure of the left atrial appendage comparable to anticoagulants for atrial fibrillation?
Abstract
For most atrial fibrillation patients oral anticoagulation constitutes the standard treatment to prevent stroke. However, they carry a risk of bleeding, which is why alternative treatments have been put into practice, such as percutaneous closure of the left atrial appendage. It is not clear whether this is as effective as the conventional treatment with anticoagulants. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including only one pertinent randomized controlled trial. We combined the evidence and generated a summary of findings following the GRADE approach. We concluded that percutaneous left atrial appendage occlusion may decrease stroke and mortality, but the certainty of the evidence is low. The effect on other outcomes is not clear because the certainty of the evidence is very low.
Problem
Stroke is one of the most serious complications of atrial fibrillation. The left atrial appendage is the source of more than 90% of clots in the case of patients with non-valvular atrial fibrillation, which is why the percutaneous closure of the left atrial appendage has become an interesting alternative to the conventional treatment with oral anticoagulants. Despite being a minimally invasive procedure, it may present complications such as severe bleeding and pericardial effusion, among others.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found three systematic reviews [1],[2],[3] including only one randomised controlled trial [4]. |
|
What types of patients were included |
Mean age of patients was 71 years old, with a CHADS2 score for atrial fibrillation stroke risk of 2.2. None of the patients had contraindication to oral anticoagulation. |
|
What types of interventions were included |
This study compared the percutaneous closure of the atrial appendage with warfarin treatment. Patients allocated to the intervention group received percutaneous closure of the left atrial appendage by use of the WATCHMAN device. This device is a self-expanding nickel titanium frame structure with a permeable polyester fabric cover, ranged in diameter from 21 mm to 33 mm. The implantation was guided by flouroscopy and transesophageal echocardiography to verify proper positioning and stability. After the device had been implanted, patients were treated with warfarin for 45 days to facilitate device endothelialization. After stopping warfarin treatment, once daily clopidogrel and aspirin were prescribed until completion of the 6-month follow up visit, from which point aspirin alone was continued indefinitely. For the control group, plasma warfarin concentration was in the therapeutic INR range (between 2.0 and 3.0) 66% of the time. |
|
What types of outcomes |
Primary efficacy (ischemic stroke, hemorrhagic stroke, cardiovascular/unexplained death, systemic embolism), any stroke, all-cause mortality. Primary safety: events related to excessive bleeding (i.e. intracranial or gastrointestinal bleeding) or procedure-related complications (i.e. serious pericardial effusion, device embolization, procedure-related stroke). Mean follow-up per patient was 18 months. |
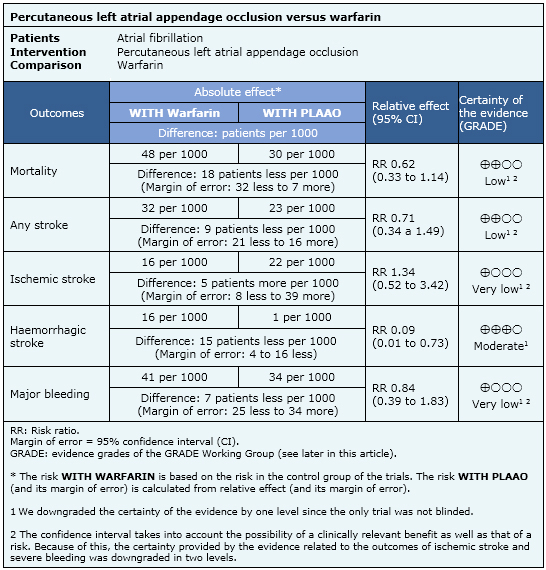
Summary of findings
The information on the effects of percutaneous left atrial appendage closure is based on one randomized controlled trial including 707 patients.
- Percutaneous left atrial appendage closure may decrease risk of stroke in comparison to oral anticoagulant treatment. The certainty of the evidence is low.
- Percutaneous left atrial appendage closure may decrease the mortality in comparison with oral anticoagulant treatment. The certainty of the evidence is low.
- It is not clear if percutaneous left atrial appendage closure increases or decreases isquemic stroke in comparison to oral anticoagulant treatment because the certainty of the evidence is very low.
- Percutaneous left atrial appendage closure probably decreases hemorrhagic stroke in comparison to oral anticoagulant treatment. The certainty of the evidence is moderate.
- It is not clear if percutaneous left atrial appendage closure increases or decreases major bleeding because the certainty of the evidence is very low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
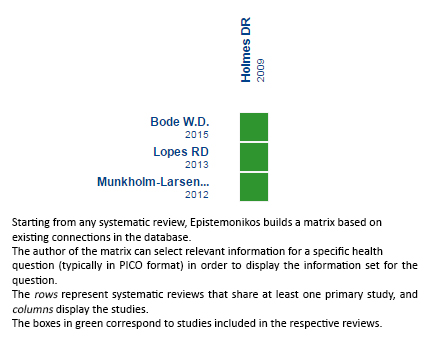 Full size
Full size Follow the link to access the interactive version: Percutaneous atrial appendage occlusion versus warfarin for atrial fibrillation
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

