Resúmenes Epistemonikos
← vista completaPublicado el 27 de octubre de 2015 | http://doi.org/10.5867/medwave.2015.6291
¿Debe indicarse terapia antitripanosómica a pacientes con enfermedad de Chagas en fase crónica?
Should trypanocidal therapy be used to treat patients in the chronic phase of Chagas disease?
Abstract
Antiparasitic treatment of patients with Chagas’ disease in chronic stage could prevent the complications related to the disease. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified five systematic reviews including eight randomized trials and 11 observational studies. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded it is not clear whether antiparasitic treatment improves survival or reduces complications related to chronic Chagas’ disease because the certainty of the evidence is very low.
Problem
Chagas' disease represents the third largest parasitic disease burden globally and the first in Latin America [1]. Chronic Chagas' cardiomyopathy is the most common form of non-ischemic cardiomyopathy worldwide, and one of the leading causes of morbidity and death in Latin America [2]. Chagas' disease has two clinical phases, acute infection that may be manifested by a self-limited febrile illness that lasts 4 to 8 weeks, and chronic phase characterized by an indeterminate stage in which patients are asymptomatic and free of complications that can last their whole life [3]. Ten to thirty years after the acute infection, about one third of the patients in chronic phase of Chagas’ disease develop cardiac or digestive complications. At the present time, the mechanism responsible for the mentioned complications is believed to be related to the presence of chronic parasitemia and its corresponding inflammatory reaction [4]. In this context, antiparasitic treatment for patients with Chagas’ disease in the chronic phase is proposed as a measure to prevent the disease’s visceral complications and their consequences.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [5],[6],[7],[8],[9] considering 19 primary studies [10],[11],[12],[13], |
|
What types of patients were included |
Patients with Chagas’ disease in chronic phase indeterminate stage or chronic phase with visceral compromise. |
|
What types of interventions were included |
Benznidazole, nifurtimox, allopurinol or itraconazole for 30 to 60 days. |
|
What types of outcomes |
Death from any cause; cardiac disease progression; congenital transmission; electrocardiographic abnormalities; serological tests negativization; xenodiagnosis negativization; polymerase chain reaction negativization; adverse effects leading to treatment discontinuation |
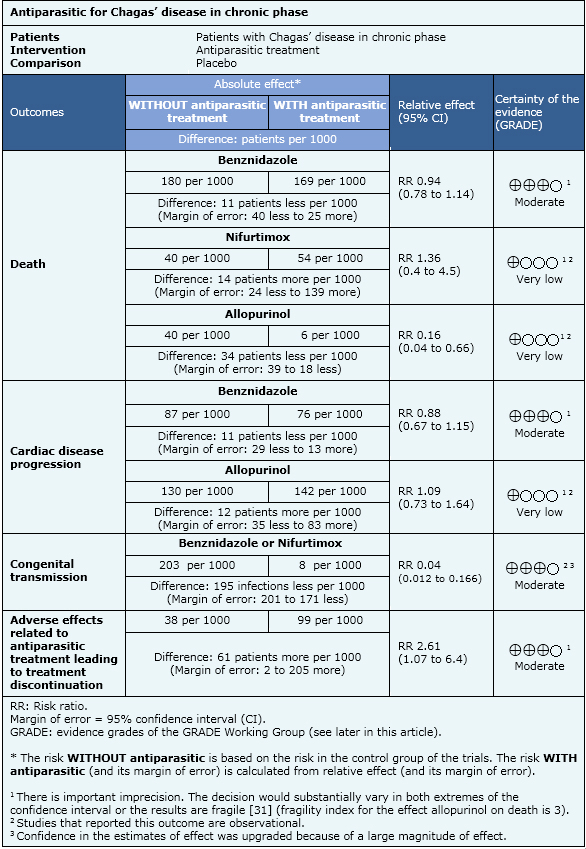
Summary of findings
The information about the antiparasitic effects is based on eight randomized controlled trials and eleven observational studies that included 7772 patients. Eight studies (one randomized and seven observational studies) reported death from any cause, six studies (one randomized and five observational studies) reported cardiac disease progression, one observational study reported congenital transmission, eleven studies (six randomized and five observational studies) reported adverse effects that lead to treatment discontinuation, four studies (three randomized and one observational study) reported electrocardiographic abnormalities, ten studies (three randomized and seven observational studies) reported serological tests negativization, four studies (two randomized and two observational studies) reported polymerase chain reaction negativization and seven studies (four randomized and three observational studies) reported xenodiagnosis negativization.
- Benznidazole therapy probably does not improve survival in patients with chronic Chagas’ heart disease. It is no clear whether other antiparasitic drugs affect survival or cardiomyopathy progression because the certainty of the evidence is very low.
- Benznidazole therapy probably does not reduce cardiomyopathy progression in patients with chronic Chagas’ heart disease. It is no clear whether other antiparasitic drugs affect cardiomyopathy progression because the certainty of the evidence is very low.
- Trypanocidal therapy probably reduces the risk of congenital transmission in young women with chronic Chagas’ disease. The certainty of the evidence is moderate.
- It is not clear whether antiparasitic treatment improves survival or reduces complications related to chronic Chagas’ disease in patients with early stages of chronic Chagas’ disease (no visceral involvement) because the certainty of the evidence in this scenario is very low.
- Antiparasitic treatment probably increases the risk of adverse effects that leads to treatment discontinuation. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
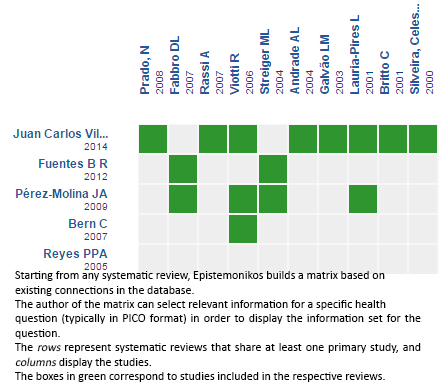
Follow the link to access the interactive version: Trypanocidal therapy to prevent chagasic cardiomyopathy
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

