Epistemonikos summaries
← vista completaPublished on November 17, 2015 | http://doi.org/10.5867/medwave.2015.6315
Is epidural steroid injection effective for degenerative lumbar spinal stenosis?
¿Sirve la infiltración epidural interlaminar de corticoides para la raquiestenosis lumbar degenerativa?
Abstract
There are several nonsurgical alternatives to treat radicular pain in degenerative lumbar spinal stenosis. Epidural steroid injections have been used for several decades, but the different studies have shown variable effects. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified nine systematic reviews including seven pertinent randomized controlled trials. We concluded epidural steroid injection probably leads to little or no effect on reducing radicular pain of spinal stenosis.
Problem
Degenerative lumbar spinal stenosis is a condition in which the area of the spinal canal decreases because of disc degeneration and facet joint osteoarthritis, predominantly in people over 65 years old. Its main symptom is intermittent neurogenic claudication, which restricts the possibility of walking due to pain in the extremities, causing a significant deterioration in the quality of life.
Among the nonsurgical alternatives epidural steroid injection is often used in order to achieve symptomatic relief, improve functionality and possibly avoid surgery. Among its risks are radicular injury, post puncture headache, metabolic disorders, rash, insomnia, among others [1].
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found nine systematic reviews [2],[3],[4],[5],[6],[7],[8],[9],[10] considering eight primary studies [11],[12],[13],[14],[15],[16],[17],[18], including seven randomized controlled trials [11],[12],[13],[15],[16],[17],[18]. This table and the summary in general are based on the latter. Only one of seven randomized trials presented data that could be included in the summary of findings [13]. The remaining studies were only used for the considerations for decision-making. |
|
What types of patients were included |
Five studies included patients with radicular pain caused exclusively by lumbar spinal stenosis [12],[13],[15],[17],[18], while two studies included patients with radicular pain due to spinal stenosis or lumbar herniated nucleus pulposus [11],[16]. |
|
What types of interventions were included |
The seven studies included in this summary used as intervention interlaminar epidural injection of steroids [11],[12],[13],[15],[16],[17],[18]. Two studies did not mention which corticosteroid was used [12],[17], three studies used methylprednisolone [11],[16],[18], one betamethasone [13] and one triamcinolone [15]. Of the three studies using methylprednisolone one administered 40 mg [18], while the other two used 80 mg [11],[16]. The study using betamethasone administered 6 mg [13], while the study with triamcinolone used 60 mg [15]. Four studies did not mention how many injections they used [12],[13],[15],[16], and the other three used two or more injections [11],[17],[18]. Five studies compared against placebo using a single injection in the same place with local anesthetics [11],[13],[16],[17],[18]. One study compared against a group without intervention [15] and one study did not make clear which intervention was used [12]. |
|
What types of outcomes |
The outcomes measured were reduction of lower extremity pain in a visual analogue scale (VAS) score, and change in disability with the Roland Morris Disability Questionnaire (RMDQ) and the Oswestry Disability Index (ODI). The time in which the effect was measured varied in different studies, ranging from one week to 4 years after the first intervention. However, meta-analysis considered endpoints at 12-weeks. |
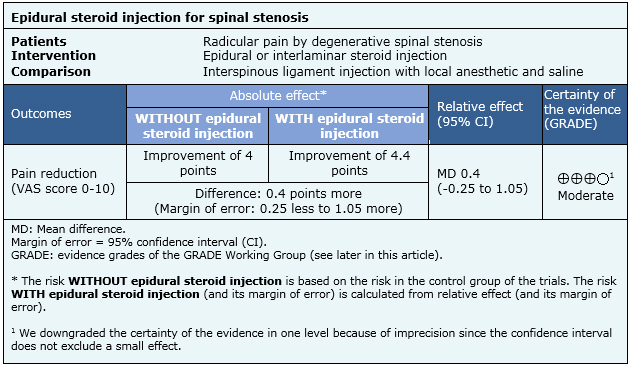
Summary of findings
The information on the effects of epidural steroid injections is based on only one study that adequately reported the outcome reduction of pain, which includes 60 patients [20].
- Epidural steroid injection probably leads to little or no effect on reducing radicular pain by spinal stenosis. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
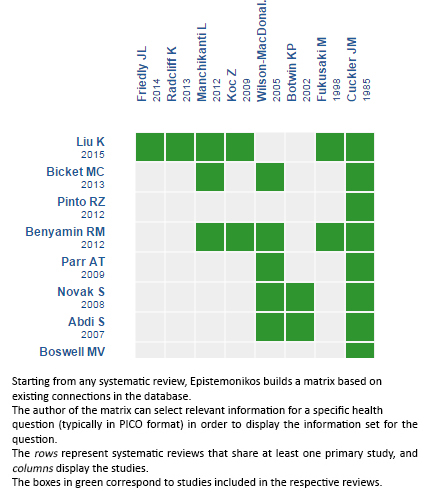
Follow the link to access the interactive version: Epidural steroid injection for degenerative lumbar spinal stenosis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

