Resúmenes Epistemonikos
← vista completaPublicado el 24 de junio de 2016 | http://doi.org/10.5867/medwave.2016.6476
¿Vale la pena utilizar dosis altas de inhibidores de la bomba de protones en la hemorragia péptica aguda?
Are higher doses of proton pump inhibitors better in acute peptic bleeding?
Abstract
Although there is broad consensus about the benefits of proton pump inhibitors in acute upper peptic bleeding, there is still controversy over their optimal dosing. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified six systematic reviews including 27 randomized trials addressing this question. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded high-dose proton pump inhibitors probably result in little or no difference in re-bleeding rate or mortality. The risk/benefit and cost/benefit balance probably favor use of low-doses.
Problem
Acute upper peptic bleeding is a serious clinical problem often requiring management in critical care units, and is associated with important morbidity and mortality.Proton pump inhibitors effectively block gastric acid secretion, favoring the healing of ulcer and halting of bleeding. The benefits of these drugs in acute upper peptic bleeding are widely recognized, but there is still controversy over their optimal dosing in this setting.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] that included 27 randomized controlled trials [7],[8],[9], [10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [21],[22],[23],[24],[25],[26],[27],[28],[29],[31],[32],[33]. |
|
What types of patients were included |
All of the studies included adult patients hospitalized for acute upper peptic bleeding. In 19 studies all patients achieved endoscopic hemostasis prior to receiving proton pump inhibitors [7],[8],[11],[13],[14], [15],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27], [28],[31],[32]. In six studies prior endoscopic hemostasis was achieved in some but not all patients [10],[12],[16],[25],[30],[33]. In two studies the information about endoscopic hemostasis was not available [9],[29]. In 14 studies ulcer characteristics were used as inclusion criteria. Thirteen studies exclusively included patients with bleeding secondary to Forrest IA to IIB ulcers [7],[8],[14],[15],[16],[17],[18],[19],[26],[27],[28],[31],[32], and only one study included Forrest IA to III ulcers [9]. |
|
What types of interventions were included |
All studies compared ‘high-dose’ proton pump inhibitors (accumulated dose of 600 mg or more in the first 72 hours) against ‘non-high’ dose (accumulated dose of less than 600 mg in first 72 hours). In all studies 'high-dose' consisted in 80 mg bolus followed by a continuous infusion of 8 mg/hour for at least 72 hours. In 18 studies ’non-high’ dose was administered by intermittent intravenous bolus [7],[8],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [22],[24],[26],[28],[30],[31]. In eight studies it was administered orally [9],[15],[21],[23],[25],[27],[29],[32], while only one study used a low dose continuous infusion [33]. |
|
What types of outcomes |
The systematic reviews identified pooled the following outcomes:
|
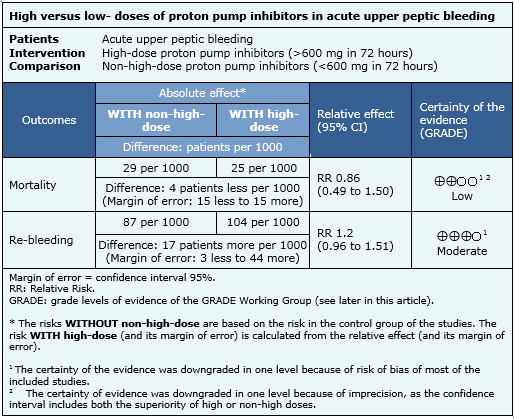
Summary of findings
The information about the effects of different doses of proton pump inhibitors in acute upper peptic bleeding is based on 21 of 27 randomized studies identified, including 2565 patients. Thirteen studies evaluated mortality [7],[9],[10],[13],[16],[19],[21],[25],[26],[29],[30],[31],[33] and 21 studies evaluated re-bleeding [7],[8],[9],[10],[13],[14],[15],[16],[17],[18],[19],[21],[25],[26],[27],[28],[29],[30],[31],[32],[33]. In the remaining six studies the data could not be extracted for meta-analysis [11],[12],[20],[22],[23],[24]. The summary of the results is the following:
- High dose proton pump inhibitors may result in little or no difference in mortality. The certainty of the evidence is low.
- High dose proton pump inhibitors probably result in little or no difference in re-bleeding risk. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
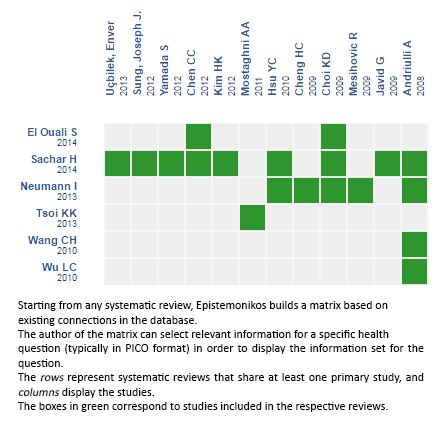
Follow the link to access the interactive version: High-dose versus non-high-dose proton pump inhibitors for bleeding peptic ulcer
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

