Resúmenes Epistemonikos
← vista completaPublicado el 5 de agosto de 2016 | http://doi.org/10.5867/medwave.2016.6499
¿Son los nuevos antibióticos superiores a los betalactámicos para los pacientes hospitalizados, no críticos, con neumonía adquirida en la comunidad?
Are new antibiotics better than beta-lactams for non-critical inpatients with community-acquired pneumonia?
Abstract
Treatment for community-acquired pneumonia in immunocompetent adults is mainly empirical. Beta-lactam antibiotics have been traditionally considered first-line therapy. New antibiotics could be more effective but the evidence is not clear until now, and its use could entail greater costs, an increase in bacterial resistance and other adverse effects. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified six systematic reviews including 36 randomized trials addressing this question. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded new antibiotics are not better than beta-lactam antibiotics for the treatment of non-critical inpatients with community-acquired pneumonia in relation to clinical failure or adverse effects.
Problem
Community-acquired pneumonia is a highly prevalent disease which affects people of all ages, carrying a high morbimortality and costs, especially at extreme ages of life. Since decades, beta-lactams have been considered the first-line of empirical therapy. With the arrival of new families of antibiotics, like macrolides, azalides, ketolides and quinolones, the use of beta-lactams as first-line therapy has been put into question and the use of new antibiotics has increased. In this context, it becomes necessary to assess the effects of beta-lactams in comparison with the alternatives.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6], including 36 randomized controlled studies reported in 37 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16], [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27], [28],[29],[30],[31],[32],[33],[34],[35],[36],[37],[38], [39],[40],[41],[42],[43]. One of the studies is reported in two papers [25],[39]. |
|
What types of patients were included |
Most of the studies included only patients with community-acquired pneumonia. One study included 10% of patients with health care-associated pneumonia [17], and five studies also included a variable percentage of patients with different respiratory conditions [15],[22],[26],[36],[41]. Thirteen studies included only inpatients [11],[14],[17], [19],[21],[22],[24],[27],[28],[36],[37],[42],[43], eight studies included inpatients and outpatients [13],[15],[18],[20],[32],[33],[39],[40], and sixteen studies did not report place of management [7],[8],[9],[10],[12],[16],[23],[25], [26],[29],[30],[31],[34],[35],[38],[41]. Seven studies included critical patients [7],[10],[19],[20], [31],[32],[37] and 23 studies did not report the severity of illness [8],[9],[11],[13],[15],[16],[17],[21],[22],[23],[24], [26],[29],[30],[33],[34],[35],[36],[38],[40],[41],[42],[43]. |
|
What types of interventions were included |
In five studies the beta-lactam used was a combination of penicillin with a beta-lactamase [13],[21],[32],[33],[34], eleven studies used a cephalosporin[8],[16],[18],[24], [26],[28],[29],[31],[37],[42],[43], three studies considered the use of a beta-lactam with a beta-lactamase inhibitor or a cephalosporin [19],[20],[27], and the remaining seventeen studies used a penicillin. Only one study used imipenem [17]. Three studies admitted the simultaneous use of a beta-lactam with a different antibiotic [18],[19],[20]. In seven studies the comparison used was a macrolide [12],[21],[23],[29],[35],[41],[43], one study compared with a quinolone or a macrolide [33] and the remaining 29 studies compared only with a quinolone. |
|
What types of outcomes |
The outcomes reported by the identified systematic reviews were: Mortality, mortality with intention-to-treat, mortality in older than 65 years, clinical success, clinical success with intention-to-treat, clinical failure, clinical failure with intention-to-treat, clinical failure in pneumococcal pneumonia, clinical failure in atypical pneumonia, clinical failure in pneumonia caused by Legionella pneumophila, microbiological success, microbiological failure, adverse effects, gastrointestinal adverse effects, adverse effects that requires discontinuation of therapy, serious adverse effects, adverse effects with intention-to-treat, and length of hospital stay. |
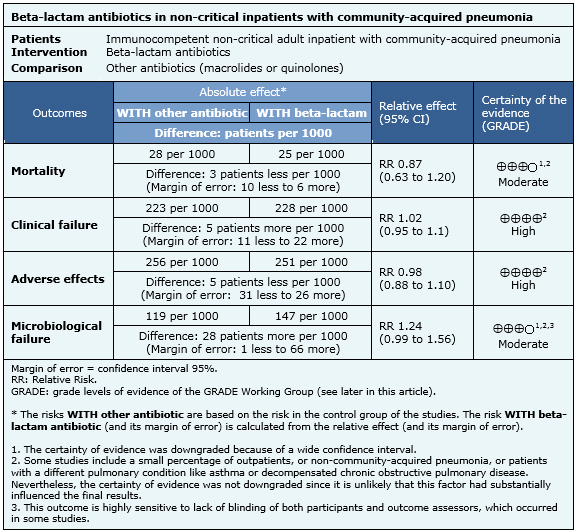
Summary of findings
The information about the effects of beta-lactam antibiotics for the treatment of non-severe community-acquired pneumonia is based on 36 randomized trials including 11,662 patients. Nineteen studies reported mortality [11],[12],[14],[15],[17],[21],[22],[27],[28],[31],[32],[33],[36],[37],[39],[40],[41],[42],[43], 32 reported clinical failure [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[21],[22],[23],[24],[26],[27],[29],[30],[31],[32],[33],[34],[35],[36],[37],[38],[39],[40],[41],[42],[43], 18 reported microbiological failure [11],[14],[15],[17],[21],[22],[24],[30],[31],[32],[33],[36],[37],[39],[40],[41],[42],[43] and 23 studies reported adverse effects [[11],[12],[13],[14],[15],[17],[18],[19],[20],[21],[22],[27],[28],[30],[31],[32],[36],[37],[39],[40],[41],[42],[43]. The summary of findings is the following:
- Beta-lactam antibiotics in non-critical adult inpatients with community-acquired pneumonia probably leads to no difference in mortality when compared to the new antibiotics. The certainty of the evidence is moderate.
- New antibiotics are not better than beta-lactam antibiotics for non-critical adult inpatients with community-acquired pneumonia in relation to clinical failure. The certainty of the evidence is high.
- New antibiotics are not better than beta-lactam antibiotics for non-critical inpatients with community-acquired pneumonia in relation to adverse effects. The certainty of the evidence is high.
- The use of beta-lactam antibiotics in non-critical adult inpatients with community-acquired pneumonia probably leads to no difference in microbiological failure when compared to the new antibiotics. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
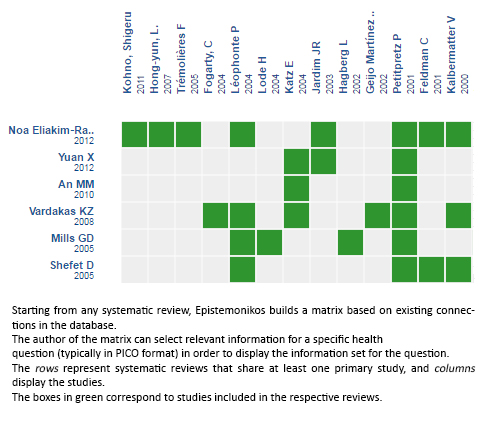
Follow the link to access the interactive version: Beta lactam antibiotics compared with non-beta lactam antibiotics for non-severe community acquired pneumonia in adults requiring hospitalization
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

