Epistemonikos summaries
← vista completaPublished on August 17, 2016 | http://doi.org/10.5867/medwave.2016.6512
Is there a role for glutamine supplementation in the management of acute pancreatitis?
¿Tiene algún rol la suplementación con glutamina en el manejo de la pancreatitis aguda?
Abstract
There is no consensus about the effects of glutamine supplementation for acute pancreatitis. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified 15 systematic reviews including 31 randomized controlled trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded glutamine supplementation might decrease infectious complications in acute pancreatitis, but it is not clear if it affects mortality or length of hospital stay because the certainty of the evidence is very low.
Problem
Glutamine is an amino acid required for nucleotide synthesis, which makes it an important energetic substrate for cells with fast turnover, such as intestinal epithelium.
Given plasmatic glutamine is reduced in critical care patients and in those undergoing major surgery, it is considered an essential amino acid under stress.
Acute pancreatitis constitutes a potentially severe condition, in which these mechanisms are particularly relevant. So, a potentially beneficial effect of glutamine supplementation has been proposed. However, it is not clear if it leads to clinically relevant effects.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 15 systematic reviews [1],[2],[3],[4],[5],[6], [7],[8],[9],[10],[11],[12],[13],[14],[15] including 31 randomized controlled trials reported in 32 references [16],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26], [27],[28],[29],[30],[31],[32],[33],[34],[35],[36],[37], [38],[39],[40],[41],[42],[43],[44],[45],[46],[47] (one study is reported in two references [30],[31]). |
|
What types of patients were included |
The different trials included patients with severe acute pancreatitis classified according to the following criteria: Glasgow in two trials [17],[21], APACHE II in eight trials [19],[20],[25],[28],[29],[30],[38],[42],[46], RANSON in six trials [19],[24],[32],[38],[46],[47], severity index according to imagenologic criteria in four [19],[28],[38], [46], ATLANTA criteria in two [26] y [27], in one trial it was not reported what criteria was used [36].Four trials used a combination of the criteria above [19],[28],[38], [46]. It was not possible to extract the information about the severity criteria used in 13 trials from any systematic review identified. |
|
What types of interventions were included |
Eight trials evaluated glutamine supplementation associated to parenteral nutrition [17],[18],[19],[20],[25], [28],[32],[37], and 10 to enteral nutrition [21],[22],[23], [24],[26],[27],[29],[30],[34],[38]. Sixteen trials used glutamine alone [17],[18],[19],[20], [21],[25],[28],[32],[36],[37],[38],[39],[42],[45],[46],[47] and eight glutamine associated to other elements [22], [23],[24],[26],[27],[29],[30],[34]: six with arginine [22], [23],[24],[26],[29],[30], one with con tributyrin and antioxidants [29], two with fiber [22],[23], one with omega-3 [30],[31] and one with Bifidobacterium, Lactobacillus and Enterococcus [34]. It was not possible to extract the information about the glutamine supplementation used in eight trials from any systematic review identified. All of the studies compared against placebo or standard treatment. |
|
What types of outcomes |
The main outcomes meta-analysed in the different reviews were: - Mortality (pooled in 13 systematic reviews). Other outcomes analysed in the different review were: plasma albumin level, plasma C-reactive protein level, surgical intervention rate, time until amylase returned to normal, hospitalization expenses, organ failure, length of intensive care unit stay, days on mechanical ventilation, feeding intolerance, systemic inflammatory response syndrome, change in white blood cells count, ventilator-associated pneumonia, abdominal pain, serious adverse events, side effects (ALT, AST, creatinine), serum amylase in patients undergoing ERCP (after 8 and 24 hours), pancreatitis in patients undergoing ERCP. The following subgroups were analysed in the different reviews: - Effect of high/low doses (more or less than 4.2 g/kg) on mortality in critical and surgical patients - Parenteral versus enteral glutamine. |
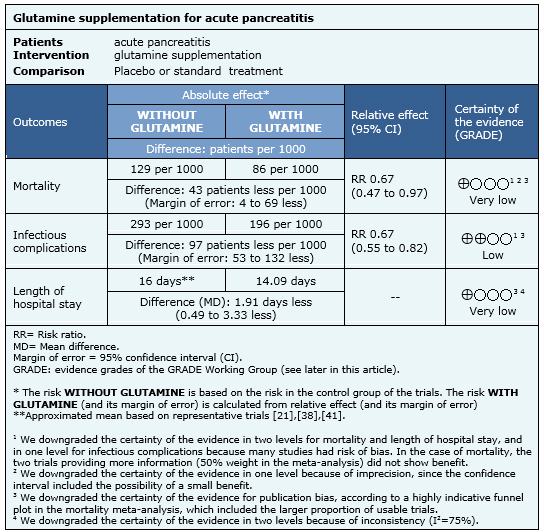
Summary of findings
Information on the effects of glutamine for acute pancreatitis is based on 22 randomized controlled trials involving 1107 participants (nine trials did not provide data for meta-analysis). All of the trials reported mortality [16],[17],[18],[19],[20],[21],[22],[24],[25],[26],[27],[28],[29],[30],[32],[34],[35],[36],[38],[39],[41],[43], 20 trials (1063 participants) reported the outcome infectious complications [16],[17],[18],[19],[21],[22],[24],[25],[26],[27],[28],[29],[30],[32],[35],[36],[39],[41],[43],[45] and 18 trials (1018 participants) reported the outcome length of hospital stay[16],[18],[19],[21],[26],[27],[28],[29],[30],[32],[34],[35],[36],[38],[39],[41],[42],[43],[44].
The summary of findings is the following:
- It is not clear whether glutamine supplementation decreases mortality because the certainty of the evidence is very low.
- Glutamine supplementation might decrease infectious complications in acute pancreatitis. The certainty of the evidence is low.
- It is not clear whether glutamine supplementation decreases length of hospital stay because the certainty of the evidence is very low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
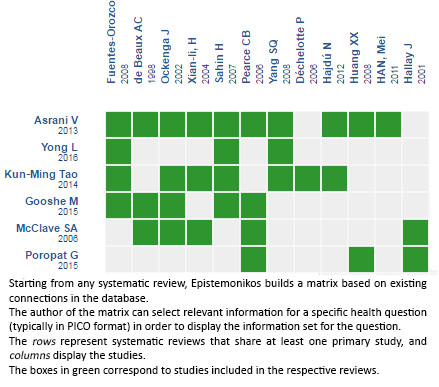
Follow the link to access the interactive version: Glutamine supplementation for acute pancreatitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

