Resúmenes Epistemonikos
← vista completaPublicado el 30 de agosto de 2016 | http://doi.org/10.5867/medwave.2016.6522
¿Cuál es el rol de los corticoides en el manejo de la sepsis?
What is the role of corticosteroids in the management of sepsis?
Abstract
During an episode of sepsis, the systemic inflammatory response phenomenon triggers a deficit in the action and/or secretion of cortisol. It has been suggested that the use of corticosteroids may have a role in the management of sepsis, but there is no consensus. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified 16 systematic reviews including 66 randomized controlled trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded the use of corticosteroids during a sepsis episode probably favors reversal of shock, briefly shortens the stay in intensive care unit and might reduce mortality, with few clinically relevant adverse effects.
Problem
Sepsis remains the leading cause of morbidity and mortality in intensive care units worldwide. Its incidence has been increasing, with more complications and more resistant infectious agents. While there has been a tendency to a decrease in mortality due to some interventions, effective therapeutic tools remain limited.
During an episode of sepsis, systemic inflammatory response phenomenon triggers a deficit in action and/or cortisol secretion secondary to proinflammatory cytokines. For this reason, it has been postulated the use of steroids may play a role in the management of sepsis. However, evidence has been mixed regarding its actual effect, and there is still no consensus on the role played by this treatment in sepsis.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found sixteen systematic reviews [1],[2],[3],[4],[5], [6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16], including 66 primary studies (64 randomized controlled trials). Two systematic reviews were excluded from the analysis. One was exclusively focused on pediatric population (whose protocols and evolution differs regarding adult population) [9] and the other was restricted to patients with dengue [6]. The remaining reviews include fifty-three randomized controlled trials [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[36],[37], [38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49], [50],[51],[52],[53],[54],[55],[56],[57],[58],[59],[60],[61], [62],[63],[64],[65],[66],[67],[68],[69]. |
|
What types of patients were included |
Nineteen studies included were conducted prior to 1991, when the first Consensus Definitions for Sepsis and Septic Shock [70] was released. So, the definition and classification of the severity of sepsis was heterogeneous among studies, added to the fact the third Consensus Definitions for Sepsis and Septic Shock has been published recently [71]. |
|
What types of interventions were included |
The most commonly used steroid was hydrocortisone (53.8%), then dexamethasone (21.1%), methylprednisolone (17.3%) and others (e.g. betamethasone, prednisolone; 7.8%). The route of administration was intravenous in all of the studies. Regarding the dose (equivalent to hydrocortisone), doses ranged from 30 mg to 4200 mg per day. The different systematic reviews used different definitions to consider low-dose corticosteroids. Some used a dose equivalent <300 mg hydrocortisone per day, another 400 mg and even 500 mg. 50% of the studies used less than or equal to 300 mg daily dose. Only two studies added mineralocorticoids [18],[28]. All of the studies compared against placebo or standard treatment. |
|
What types of outcomes |
The different systematic reviews pooled the following outcomes: - Mortality at 28 days |
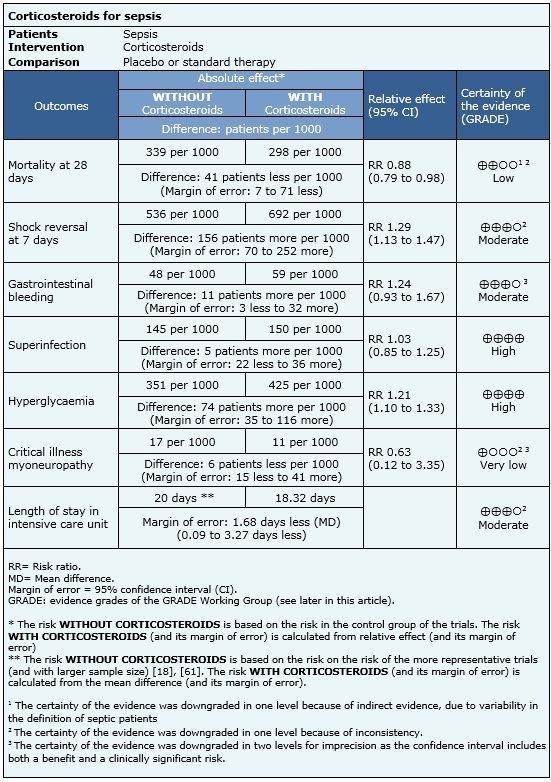
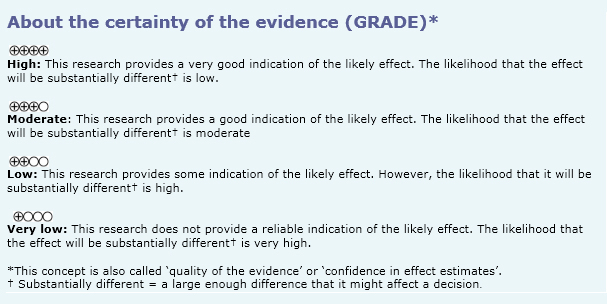
Summary of findings
Information on the effects of using steroids in sepsis is based on 44 randomized trials that provided information usable for meta-analysis involving 5618 patients. Forty-four trials reported mortality [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[29],[30],[32],[33],[34],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[51],[52],[53],[55],[56],[57],[58],[59],[60],[61],[62],[63],[64],[65],[66],[67],[68],[69], 12 trials reported reversal of shock [18],[19],[22],[23],[24],[25],[32],[34],[55],[57],[60],[61], 12 trials reported stay in intensive care [18],[19],[22],[24],[25],[29],[32],[34],[44],[59],[61],[66], 20 trials reported gastrointestinal bleeding [18],[19],[20],[22],[24],[25],[27],[29],[32],[42],[44],[46],[55],[57],[59],[61],[63],[67],[68],[69], 21 trials reported superinfection [17],[18],[19],[20],[22],[23],[24],[25],[27],[29],[32],[39],[42],[46],[57],[59],[60],[61],[63],[68],[69], 13 trials reported hyperglycemia [18],[19],[22],[42],[44],[46],[57],[59],[60],[61],[63], [68],[69] and three trials reported myoneuropathy of critically ill patients [18],[19],[61]. The summary of findings is the following:
- The use of corticosteroids in patients with sepsis might decrease mortality. The certainty of the evidence is low.
- The use of corticosteroids in patients with sepsis probably increases the chance of shock reversal at 7 days. The certainty of the evidence is moderate.
- The use of corticosteroids probably produces a slight increase in the risk of upper gastrointestinal bleeding. The certainty of the evidence is moderate.
- The use of corticosteroids in patients with sepsis increases the risk of hyperglycemia. The certainty of the evidence is high
- The use of corticosteroids in patients with sepsis has little or no effect on the risk of superinfection. The certainty of the evidence is high.
- It is unclear whether the use of corticosteroids in patients with sepsis increases the risk of critical illness myoneuropathy. The certainty of the evidence is very low.
- The use of corticosteroids probably decreases slightly the time spent in intensive care units. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
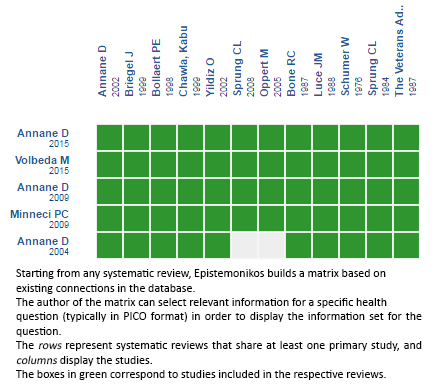
Follow the link to access the interactive version:Corticosteroids for sepsis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

