Resúmenes Epistemonikos
← vista completaPublicado el 6 de septiembre de 2016 | http://doi.org/10.5867/medwave.2016.6528
¿Tiene algún rol la erradicación de Helicobacter pylori en la trombocitopenia inmune?
Does Helicobacter pylori eradication play a role in immune thrombocytopenia?
Abstract
Helicobacter pylori infection has been implicated as trigger or disease modifier in immune thrombocytopenia (ITP). So, eradication treatment for this agent could have clinical benefits. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified four systematic reviews comprising 40 studies addressing the question of this article overall, including one randomized controlled trial. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded Helicobacter eradication might decrease risk of bleeding in patients with immune thrombocytopenia but the certainty of the evidence is low.
Problem
Helicobacter pylori is known for its influence on the development of gastrointestinal diseases. However, it could also play a role in diseases outside the digestive system such as immune thrombocytopenia (also called idiopathic or immune thrombocytopenic purpura - ITP). This condition is characterized by the presence of antibodies against platelets, and it has been postulated Helicobacter might act as a trigger, and also as a disease modulator by a mechanism not completely understood.
For this reason, it has been suggested eradication of Helicobacter could be beneficial. However, it is unclear whether this actually translates into clinical benefits.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1], [2], [3], [4], including 40 studies addressing the question of interest [5], [6], [7], [8] ,[9], [10], [11], [12],[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], |
|
What types of patients were included |
The trial included adult patients between 18 and 75, with chronic immune thrombocytopenia and Helicobacter pylori infection confirmed with urea breath test. Patients were included only if secondary causes of thrombocytopenia had been clearly ruled out, including hepatitis B or C virus infection, HIV infection, lupus, antiphospholipid antibody syndrome, bone marrow failure syndromes, drug-induced thrombocytopenia, and malignancies such as chronic lymphocytic leukemia and malignant lymphoma.Patients with renal failure, severe liver failure, allergic to amoxicillin, clarithromycin or lansoprazole and patients with platelet counts below 20 x 103/µl or above 100 x 103/µl were also excluded. |
|
What types of interventions were included |
The trial evaluated eradication of Helicobacter pylori with amoxicillin 750 mg, clarithromycin 200 mg and lansoprazole 30 mg bid for one week. The trial compared against placebo. |
|
What types of outcomes |
All of the systematic reviews evaluated only considered the outcome platelet response, measured in different ways. The randomized trial defined a platelet count of 150 x 103/µl as complete response and a partial response over 50 x 103/µl. |
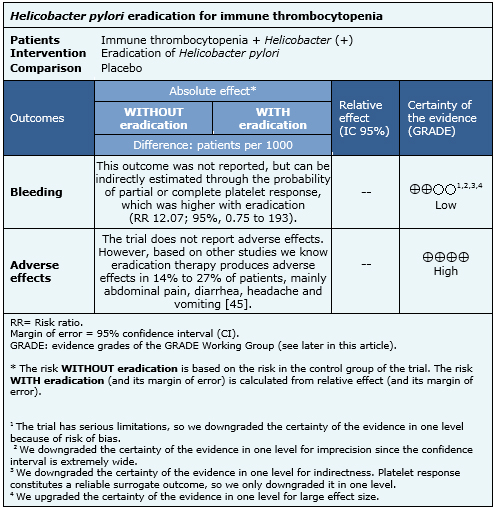
Summary of findings
The Information on the effects of Helicobacter pylori eradication in patients with immune thrombocytopenia is based in only one randomized trial including 25 participants [32]. The trial measured increase in platelet count after the eradication of Helicobacter pylori, which was used as indirect evidence of the effect on the risk of bleeding. The summary of findings is as follows:
- Helicobacter eradication might decrease bleeding risk in immune thrombocytopenia, but the certainty of the evidence is low.
- Adverse effects of Helicobacter eradication therapy are common but self-limiting and not severe. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
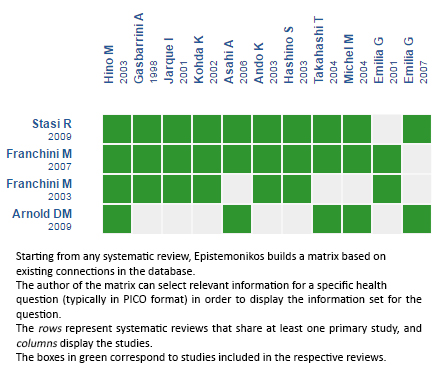
Follow the link to access the interactive version: Helicobacter pylori eradication for immune thrombocytopenia (ITP)
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

