Resúmenes Epistemonikos
← vista completaPublicado el 15 de septiembre de 2016 | http://doi.org/10.5867/medwave.2016.6545
¿Cabergolina o bromocriptina para el prolactinoma?
Cabergoline or bromocriptine for prolactinoma?
Abstract
Cabergoline and bromocriptine are among the most commonly used drugs to treat prolactinoma. Cabergoline is a long-acting dopamine receptor agonist which might offer advantages over bromocriptine. However, it is not clear if this translates into clinical benefits. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified two systematic reviews including 12 studies addressing the question of this article, including five randomized controlled trials. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded cabergoline is more effective than bromocriptine in resolution of amenorrhea/oligomenorrhea and galactorrhea, it probably increases pregnancy rate, and it is associated to less adverse effects. It is not clear whether cabergoline is also more effective with respect to tumor growth because the certainty of the evidence is very low.
Problem
Prolactinoma produces symptoms by increased prolactin secretion and as consequence of tumor enlargement. Dopamine agonists inhibit prolactin pituitary secretion by stimulation of D2 receptors, and so they control hyperprolactinemia and tumor growth.
Bromocriptine has been effectively used for decades, but it is associated to adverse effects. Cabergoline, a long-acting dopamine agonist, may be associated with less adverse effects, but it is not clear whether its pharmacological advantages translate into better clinical outcomes.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found two systematic reviews [1],[2] comprising 12 studies overall [3],[4],[5],[6],[7],[8],[9],[10],[11],[12], [13],[14], including five randomized controlled trials relevant for the question of this article [3],[9],[10],[12],[14]. This summary is based on data from the randomized trials except for outcomes that were only reported in non-randomized studies [5],[6],[11]. |
|
What types of patients were included |
One trial included patients with prolactin blood level above normal [10], two studies used a 2-fold increase above normal [3],[14], and one a 3-fold increase [12]. We did not find data in any of the reviews regarding prolactin level used as inclusion criteria for one study [9]. Three studies included women with amenorrhea for more than 3 months [3],[10],[14], and one study included women with hyperprolactinemia undergoing intrauterine insemination [9]. The three non-randomized studies correspond to retrospective cohorts. Two included men with macroprolactinoma [5] and prolactinoma (micro and macroprolactinoma) [11]. One study included patients with hyperprolactinemia without other specification in the systematic reviews identified [6]. |
|
What types of interventions were included |
All of the studies compared bromocriptine against cabergoline. Regarding bromocriptine: three trials administered doses of 5-10 mg per day [10],[12],[14]. Two studies used 5 mg per day [3],[9]. One non-randomized trial used 1.25 mg twice a day for one week, 2.5 mg twice a day for three weeks and then on according to prolactin levels [5]. Another trial used 2.5 mg pm for two weeks, then 5 mg at lunch and 2.5 mg pm, and subsequent doses were adjusted by prolactin level [6]. Regarding cabergoline: One trial administered a fixed dose of 0.25 mg twice a week [9], one trial used 0.5 mg per week [3], one study 1 mg per week [12] and two 1-2 mg per week [10],[14]. One non-randomized study used 0.5 mg once a week for 15 days, then 0.5 mg twice a week, and then according to prolactin levels [5]. Another trial used 0.25 mg once a week for one week and then 0.25 mg twice a week, then adjusted by prolactin level [6]. None of the reviews report information about cabergoline and bromocriptine doses used in one of the trials [11]. |
|
What types of outcomes |
The main outcomes meta-analysed in the different reviews were: Amenorrhea/oligomenorrhea and galactorrhea (pooled in both systematic reviews [1],[2]]. Decreased libido, increased tumor size, low testosterone, masculine infertility, pregnancy, sexual dysfunction, visual defect and prolactin level increase (pooled in only one systematic review [2]). Adverse effects (nausea, vomiting, hypotension, headache, among others) (pooled in only one systematic review [1]). |
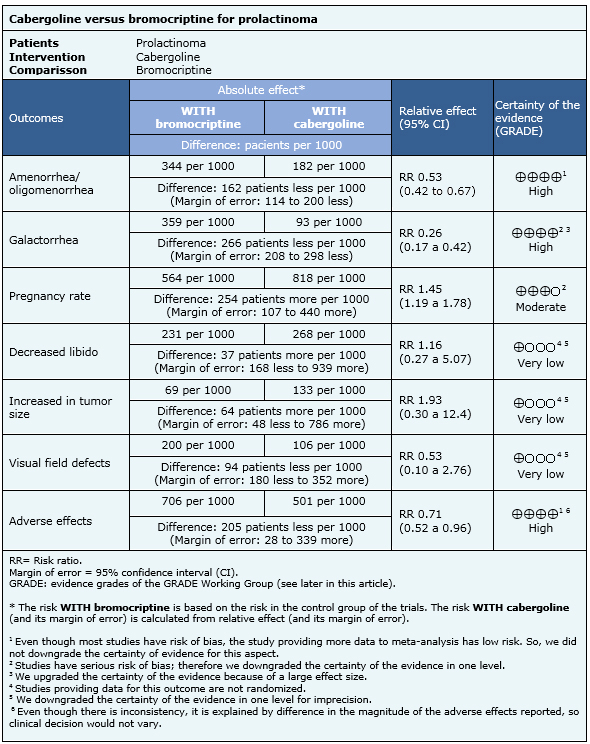
Summary of findings
The information on the effects of cabergoline versus bromocriptine is based on five randomized controlled trials involving 906 patients [3], [9], [10], [12], [14] and three non-randomized studies [5], [6, [11] which provided information about outcomes not reported in randomized trials. The five randomized trials (906 patients) measured oligomenorrhea/amenorrhea [3], [9], [10], [12], [14], four reported galactorrhea [3], [9], [12], [14], one provided information about pregnancy rates [9] and four trials reported adverse effects [3], [10], [12], [14]. Two non-randomized studies provided information on increase in libido [5], [6], one reported effects on tumor growth [11] and one addressed visual field defects [6].
The summary of findings is the following:
- Cabergoline is more effective than bromocriptine in resolving amenorrhea/oligomenorrhea. The certainty of the evidence is high.
- Cabergoline is more effective than bromocriptine in resolving galactorrhea. The certainty of the evidence is high.
- Cabergoline probably increases pregnancy rate in comparison to bromocriptine. The certainty of the evidence is moderate.
- It is not clear whether cabergoline is more effective than bromocriptine in increasing libido, because the certainty of the evidence is very low
- It is not clear whether cabergoline is more effective than bromocriptine in preventing tumor growth, because the certainty of the evidence is very low.
- It is not clear whether cabergoline is more effective than bromocriptine in diminishing visual defect, because the certainty of the evidence is very low.
- Cabergoline leads to less adverse effects than bromocriptine. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
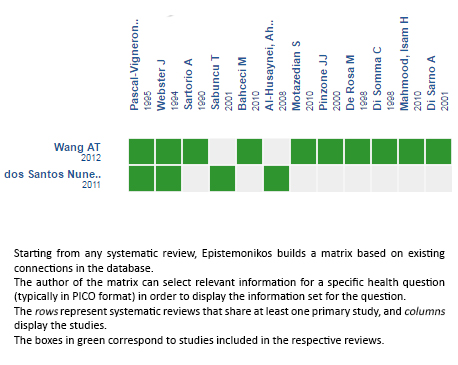
Follow the link to access the interactive version: Cabergoline versus bromocriptine for hyperprolactinemia or prolactinoma
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

