Resúmenes Epistemonikos
← vista completaPublicado el 29 de septiembre de 2016 | http://doi.org/10.5867/medwave.2016.6555
¿Es efectiva la N-acetilcisteína en el tratamiento de la fibrosis pulmonar?
Is N-acetylcysteine effective in the treatment of pulmonary fibrosis?
Abstract
Idiopathic pulmonary fibrosis is a progressive chronic respiratory disease that in final stages carries high mortality. Several treatment options have been proposed, including N-acetylcysteine, but its role is not clearly established. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified eight systematic reviews including 16 trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded N-acetylcysteine might increase the risk of hospitalizations and exacerbations. While it is unclear whether this leads to increased mortality because the certainty of the evidence is very low, in general there is consensus that it should not be used except in the context of a new clinical trial.
Problem
A substantial amount of evidence has appeared in the last years regarding different therapies potentially effective for idiopathic pulmonary fibrosis. This is not surprising since the only intervention that clearly increases survival is lung transplantation [1].
N-acetylcysteine, by its reducing character, exerts a cytoprotective activity in the human respiratory tract, acting against the harmful action of oxidative stress generated by free radicals of diverse etiology. Based on its structure derived from cysteine, N-acetylcysteine has a precursor role in the synthesis of the antioxidant molecule glutathione and normalizes its levels when they are reduced by a continuous oxidizing action on the respiratory system. This mechanism would explain a potential benefit in idiopathic pulmonary fibrosis [2].
In addition, N-acetylcysteine is widely available, and according to the ATS/ERS 2011 guideline, when used alone or in combination (with prednisolone and azathioprine) was a reasonable choice in a minority of patients with this disease [1]. However, an update of the same guideline in 2015 proposed banning its use due to an increased risk of hospitalization and death in a pivotal trial [3].
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found eight systematic reviews published in nine references [4],[5],[6],[7],[8],[9],[10],[11],[12] including 16 randomized controlled trials published in 18 references [13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23],[24], [25],[26],[27],[28],[29],[30]. |
|
What types of patients were included |
All of the trials included patients over 18 years. Another trial also required markers of lung injury [14] and in one trial diagnosis was only based on images and biopsy [18]. |
|
What types of interventions were included |
Two trials used oral N-acetylcysteine monotherapy [15], [17], one trial used inhaled monotherapy [14], ten trials used a combination with prednisone 0,4 to 0,5 mg/kg/day [13],[20],[21],[23],[24],[25],[26],[27],[30], one trial used it associated with interferon [22], one study refers it associated N-acetylcysteine to antiinflammatory treatment but does not specifies which one [29], one trial administered it associated to prednisolone and azathioprine [18], and another trial that used N-acetylcysteine associated to prednisolone and azathioprine was interrupted early due to an increase in mortality risk [16]. |
|
What types of outcomes |
The systematic reviews identified pooled outcomes as follows:
|
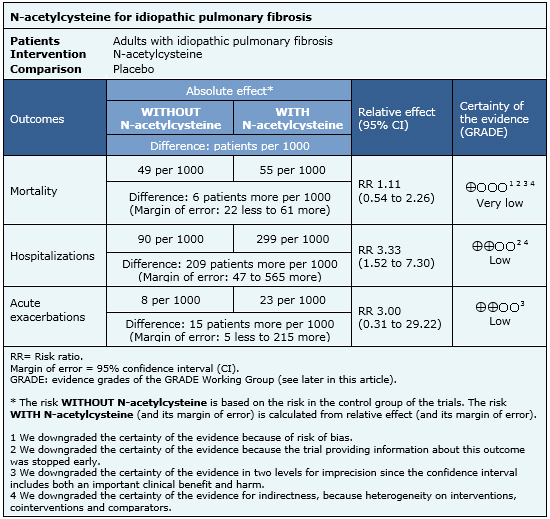
Summary of findings
The information on the effects of N-acetylcysteine is based on four randomized trials [14],[15],[16],[18] including 694 patients. The rest of the trials did not report the outcomes of interest, or did not present data in a format suitable for meta-analysis. Four trials [14],[15],[16],[18] measured the outcome mortality, and only one trial [16] reported hospitalizations and acute exacerbations. The summary of findings is as follows:
- It is unclear whether N-acetylcysteine increases or decreases mortality in idiopathic pulmonary fibrosis because the certainty of the evidence is very low.
- N-acetylcysteine might increase the risk of hospitalization in idiopathic pulmonary fibrosis, but the certainty of the evidence is low.
- N-acetylcysteine might increase the risk of acute exacerbations in idiopathic pulmonary fibrosis, but the certainty of the evidence is low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
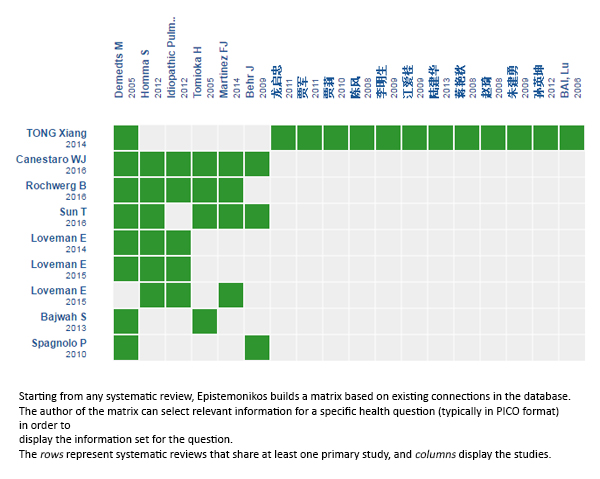
Follow the link to access the interactive version: N-acetylcysteine for idiopathic pulmonary fibrosis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

