Resúmenes Epistemonikos
← vista completaPublicado el 7 de noviembre de 2016 | http://doi.org/10.5867/medwave.2016.6591
¿Tiene algún rol la suplementación con ácido fólico en la insuficiencia renal crónica?
Is folic acid supplementation useful for chronic kidney disease?
Abstract
Patients with chronic kidney disease have higher cardiovascular risk than general population, a fact that has been linked to high homocysteine levels. Folic acid supplementation can reduce homocysteine levels, which would reduce cardiovascular events. However, there is controversy about the clinical effects of this measure. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified six systematic reviews comprising 13 trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded folic acid supplementation does not reduce the risk of myocardial infarction or stroke in patients with chronic kidney disease, and might have no effect on mortality.
Problem
It is clearly established patients with chronic kidney disease have a higher cardiovascular risk than the general population. However, despite control of classical risk factors (diabetes, hypertension, dyslipidemia, and smoking, among others) there is still a difference compared to population with these conditions without chronic kidney disease. In parallel, it has been found homocysteine levels are elevated in chronic kidney disease and there is an association with cardiovascular events and consequent mortality.
Considering folic acid can reduce homocysteine levels, it has been proposed as a potentially effective treatment to reduce cardiovascular diseases in patients with chronic kidney disease. However, there is controversy about the clinical effects of this measure.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] including 13 randomized controlled trials reported in 24 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16], [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27], [28],[29],[30],[31],[32]. |
|
What types of patients were included |
General characteristics: The average age of patients in the trials was 58.7 years. The average percentage of men was 66%. Type and stage of kidney disease: Three trials [23],[30],[32] included patients on any stage of chronic kidney disease, four trials [7],[24],[27],[29] on pre-dialysis stage, five on dialysis [22],[25],[26], [28],[31] and one after transplantation [9]. Ten trials [9],[22],[23],[25],[26],[27],[28],[29],[31],[32] included any type of kidney disease, two trials [7],[30] included only diabetic nephropathy and one trial [24] included diabetic nephropathy or vascular nephropathy. Comorbidities: The average percentage of patients with a history of acute myocardial infarction was 40.9%, including one trial that restricted inclusion to post-infarction patients [27]. The average percentage of patients with diabetes was 40.9%, with two trials using this as an inclusion criterion [7],[30]. |
|
What types of interventions were included |
Five trials [25],[28],[30],[31],[32] evaluated folic acid as monotherapy, and eight trials [7],[9],[22],[23],[24],[26],[27],[29] in combination with vitamin B6 and/or B12. The average dose of folic acid was 8.8 mg/day, ranging from 2 to 40 mg/day. Eight trials [7],[23],[24],[27],[28],[29],[30],[32] compared against placebo, two trials [25], [26] compared against usual care, one trial [9] against vitamin B and two trials [22],[31] against a lower dose of folic acid with or without vitamin B supplement. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
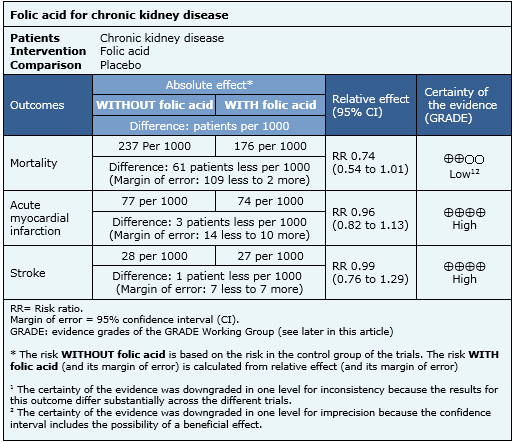
Summary of findings
The information on the effects of folic acid in patients with chronic kidney disease is based on thirteen randomized trials involving 11,049 patients.
Nine trials (8,500 patients) measured the outcome mortality [7],[9],[22],[23],[24],[26],[28],[31],[32], seven trials (7,718 patients) measured myocardial infarction [7],[9],[22], [23],[26],[31],[32] and seven trials (8,536 patients) measured stroke [9],[22],[23],[24],[26],[31],[32].
The summary of findings is the following:
- Folic acid supplementation might have no effect on mortality in patients with chronic kidney disease, but the certainty of the evidence is low.
- Folic acid supplementation does not reduce the risk of acute myocardial infarction or stroke in patients with chronic kidney disease. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
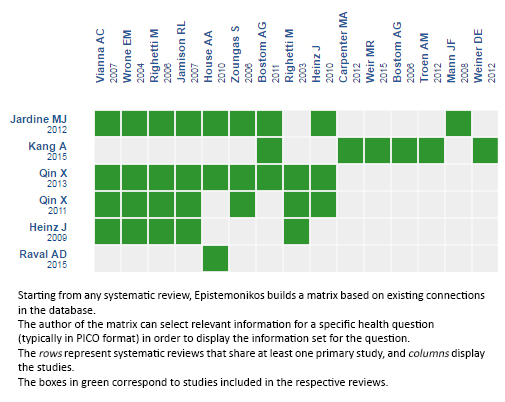
Follow the link to access the interactive version: Folic acid for chronic kidney disease.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

