Resúmenes Epistemonikos
← vista completaPublicado el 2 de diciembre de 2016 | http://doi.org/10.5867/medwave.2016.6614
¿Mejora la respuesta clínica al agregar un segundo antipsicótico a la clozapina en esquizofrenia resistente?
Does adding a second antipsychotic to clozapine improve clinical response in resistant schizophrenia?
Abstract
Clozapine constitutes the treatment of choice in patients with schizophrenia with persisting symptoms despite antipsychotics at adequate dose and treatment duration. However, an important proportion does not respond to optimal doses of clozapine, so the addition of a second antipsychotic might increase clinical response.
Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified 17 systematic reviews comprising 62 studies addressing the question of this article, including 26 randomized trials. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded adding a second antipsychotic to clozapine in patients with refractory schizophrenia probably leads to little or no difference in clinical response, and increases adverse effects.
Problem
Among 20% to 30% of patients with schizophrenia are considered to have a treatment resistant illness, namely persistent psychotic symptoms despite adequate treatment with antipsychotic drugs [1]. For these patients, clozapine is the treatment of choice [2],[3],[4]. However, an important proportion might not achieve remission despite clozapine at optimal doses [5]. So, it has been postulated adding a second antipsychotic would improve clinical response, but there is no consensus about this issue. On the other hand, this measure is associated to important adverse effects and costs.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 17 systematic reviews [5],[6],[7],[8],[9], |
|
What types of patients were included |
All of the trials included adults with a diagnosis of schizophrenia (six trials using DSM IV or CIE 10 criteria [31], [39], [44], [62], [63], [64]), with persistence of psychotic symptoms despite adequate treatment with clozapine. Nineteen trials also included conditions related to schizophrenia [22], [23],[24],[25], [31], [35], [39], [40], [41], [44], [48], [58], [62], [63], [64], [66], The trials included in- and outpatients, with no major medical or psychiatric comorbidity. In relation to severity of illness at the beginning of the study, 11 trials reported PANSS >= to 60 [23], [24], [36], [41], [43], [44], [48], [52], [58], [64], [70], 11 trials BPRS >= to 25 [22], [25], [31], [34], [35], |
|
What types of interventions were included |
All the trials compared a combination of clozapine with another antipsychotic against clozapine alone or clozapine plus placebo. Eleven trials added risperidone [23], [24], [39], [40], [43], [44], [48], [58], [62], [63], [64], five sulpiride [22], [25], [31], [70], [79], four aripiprazole [34], [37], [66], [67], two pimozide [35], [41], one pipotiazine [84], one haloperidol [52], one ziprasidone [78], one sertindole [36] and one amisulpiride [75]. |
|
What types of outcomes |
The trials measured multiple outcomes, however the different systematic reviews grouped them as follows:
|
Summary of findings
The information about the effects of adding a second antipsychotic to clozapine is based on 12 randomized trials [22], [25], [31], [39], [40], [44], [48], [58], [62], [63], [64], [79], which include 771 patients. The remaining trials did not report the outcomes of interest or did not provide data suitable for meta-analysis. The summary of findings is the following:
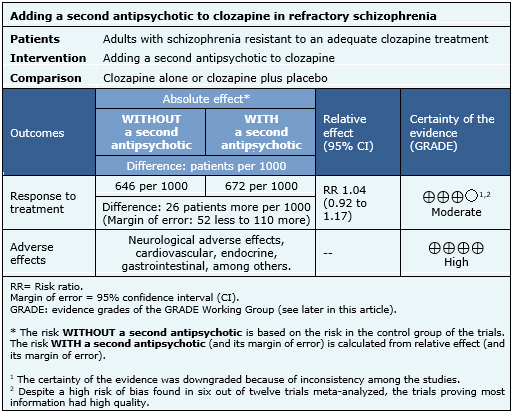
- Adding a second antipsychotic to clozapine in patients with refractory schizophrenia probably results in little or no difference in clinical response. The certainty of the evidence is moderate.
- Adding a second antipsychotic to clozapine in patients with refractory schizophrenia increases adverse effects. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
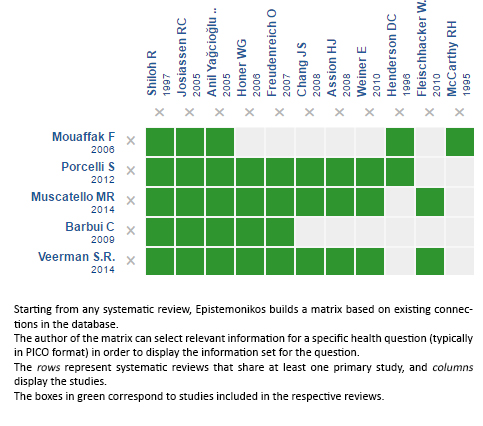
Follow the link to access the interactive version: Adding a second antipsychotic to clozapine for resistant schizophrenia
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

