Resúmenes Epistemonikos
← vista completaPublicado el 10 de marzo de 2017 | http://doi.org/10.5867/medwave.2017.6865
¿Son efectivos los cannabinoides en la esclerosis múltiple?
Are cannabinoids effective in multiple sclerosis?
Abstract
Multiple beneficial effects have been proposed lately for cannabinoids in different clinical situations. Among them, it has been postulated they would control symptoms of multiple sclerosis. However, there is no consensus about their real clinical role. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple databases. We identified 25 systematic reviews including 35 studies overall, of which 26 were randomized trials. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabinoids in multiple sclerosis do not reduce spasticity or pain, and are probably associated to frequent adverse effects.
Problem
Multiple sclerosis is a demyelinating chronic disease of the central nervous system that might presents in a relapsing-remitting and/or progressive pattern. The clinical manifestations are multiple and include loss of strength and/or sensitivity in the limbs, visual loss, pain secondary to spasticity, ataxia, and bladder dysfunction. The treatment for relapses is usually based on corticosteroids. In the long term, different therapeutic alternatives are available, such as interferon beta, immunomodulatory agents, immunoglobulin, chemotherapeutic agents and sphingosine analogues. However, up to 30-40% of patients remain symptomatic.
Multiple beneficial effects have been proposed lately for cannabinoids in different clinical situations. Among them, it has been postulated tetrahydrocannabinol and cannabinidiol would control spasticity, pain and bladder dysfunction in multiple sclerosis, especially in patients with refractory symptoms. The proposed mechanisms are mediated through modulation of CB1 and CB2 receptors in the endocannabidiol system. However, the real clinical impact of cannabinoids in this condition is not clear.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 25 systematic reviews [1],[2],[3],[4],[5],[6],[7], [8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [21],[22],[23],[24],[25], including 35 primary studies reported in 57 references [26],[27],[28],[29],[30],[31],[32],[33],[34], [35],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46], [47],[48],[49],[50],[51],[52],[53],[54],[55],[56],[57],[58], [59],[60],[61],[62],[63],[64],[65],[66],[67],[68],[69],[70], [71],[72],[73],[74],[75],[76],[77],[78],[79],[80],[81],[82], [83],[84],[85], among them 26 randomized controlled trials [26],[28],[33],[36],[38],[39],[40],[47],[48],[49],[52],[54], [57],[59],[60],[61],[62],[63],[70],[71],[73],[74],[76],[77], [79],[80]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
All of the trials included patients with multiple sclerosis, but just a few specified the subtype: six trials included patients with relapsing-remitting multiple sclerosis [39],[40],[52], [60],[73],[76],[80]; seven trials included patients with primary progressive multiple sclerosis [40],[49],[52],[65], [71],[76],[80]; and eight trials included patients with secondary progressive multiple sclerosis [28],[33],[40],[49], [52],[71],[76],[80]. The age of the included patients was reported only in some trials, with a variable range, greater than 18 years old in all the cases. The severity of the included patients was reported in only seven trials, using the EDSS score (expanded disability status scale), presenting a score greater than five in all of the trials [28],[36],[40],[49],[71],[76],[80]. In just eight trials the duration of disease was described, with a range of 4.5 to 17 years in the different trials [28],[33],[36],[49],[52],[71],[76], [80]. |
|
What types of interventions were included |
One trial administered smoked cannabis [28], nine trials used cannabis capsules [39],[40],[49],[61],[73],[74],[76],[79], [80]; two trials used dronabinol [40], [71]; and ten trials used sublingual nabiximol spray [26],[33],[36],[38],[47],[48],[52], [59],[62],[70]. Other trials used less conventional presentations. All of the trials compared against placebo. |
|
What types of outcomes |
The different systematic reviews grouped the outcomes in the following way:
|
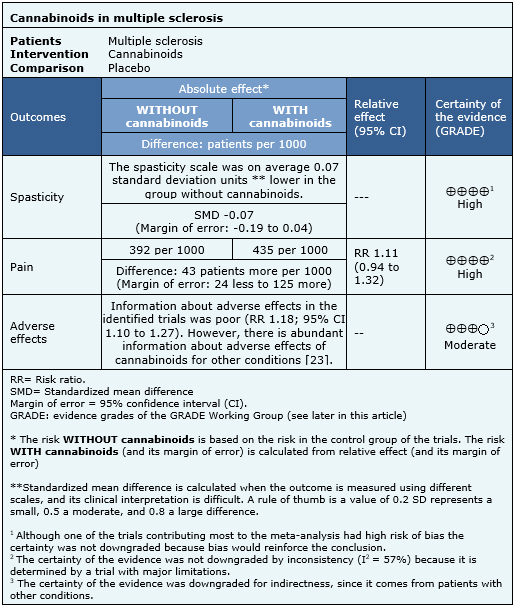
Summary of findings
The information about the effects of cannabinoids in multiple sclerosis is based on seven randomized trials [33],[35],[36],[43],[52],[63],[69], that included 1,985 patients. The other trials did not report any outcome of interest, or did not present the information in a way it could be incorporated in a meta-analysis. Four trials [33],[36],[63],[80] reported spasticity (1,247 patients), three trials [33],[52],[70] reported pain (327 patients) and four trials [33],[36],[52],[63] reported adverse effects (1,025 patients). The summary of findings is the following:
- Cannabinoids do not reduce spasticity in multiple sclerosis. The certainty of the evidence is high.
- Cannabinoids do not reduce pain in multiple sclerosis. The certainty of the evidence is high.
- Cannabinoids are associated to adverse effects, which are probably frequent in multiple sclerosis. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
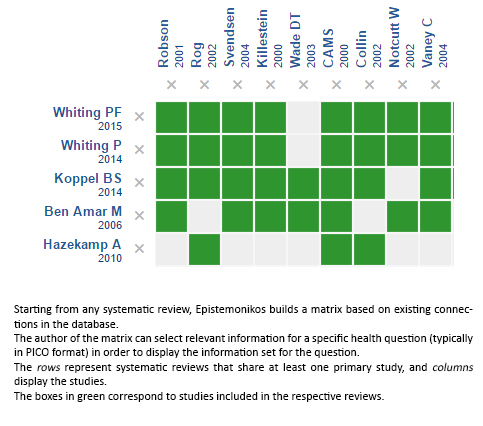
Follow the link to access the interactive version: Cannabinoids for multiple sclerosis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

