Epistemonikos summaries
← vista completaPublished on April 26, 2017 | http://doi.org/10.5867/medwave.2017.6929
Is chondroitin sulfate effective for osteoarthritis?
¿Es efectivo el condroitín sulfato para el tratamiento de la artrosis?
Abstract
Osteoarthritis is the most prevalent chronic articular disease, in which pain is one of the main symptoms and the major determinant of functional loss. Several therapeutic options have been proposed, including chondroitin sulfate, but its actual usefulness has not yet been established. To answer this question we searched in Epistemonikos database, which is maintained by screening multiple information sources. We identified 13 systematic reviews including 50 randomized trials overall. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded it is not clear whether the use of chondroitin sulfate leads to an improvement in pain or functionality in osteoarthritis because the certainty of the evidence is very low.
Problem
Osteoarthritis is the most prevalent chronic joint disease in the world, and is associated with progressive and chronic joint cartilage damage, producing pain and limiting the functionality of patients.
One of the drugs available for the management of osteoarthritis is chondroitin sulfate, which in in vitro models has beneficial effects in the metabolism of chondrocytes, synoviocytes and subchondral bone cells, increasing the synthesis of type II collagen and proteoglycan, decreasing the production of inflammatory mediators and proteases, slowing cell death and improving the anabolic-catabolic balance of the cartilage extracellular matrix. Even so, results of its effects differ in clinical trials. Chondroitin sulfate is recommended as a slow-acting drug for osteoarthritis in some international guidelines, while other guidelines do not recommend it or just for certain conditions.
Methods
We used Epistemonikos database, which is maintained by screening multiple information sources, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified 13 systematic reviews [1],[2],[3],[4],[5],[6], [7],[8],[9],[10],[11],[12],[13] that included 50 randomized controlled trials reported in 53 references [14],[15],[16], [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27], [28],[29],[30],[31],[32],[33],[34],[35],[36],[37],[38], [39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49], [50],[51],[52],[53],[54],[55],[56],[57],[58],[59],[60], [61],[62],[63],[64],[65],[66]. |
|
What types of patients were included |
Four trials included outpatients [21],[52],[54],[66]. The rest of the trials did not provide this information. Thirty eight trials included patients with knee osteoarthritis [14],[15],[17],[19],[20],[21],[22],[24],[25],[26],[27], [28],[29],[31],[38],[39],[41],[42],[43],[44],[45],[46], [48],[50],[51],[52],[53],[54],[55],[56],[57],[60],[61], [62],[63],[64],[65],[66]. Two trials included hip osteoarthritis [16] and [23], four included hand osteoarthiritis [35],[37],[40],[58], three trials included osteoarthritis in more than one joint [18],[32],[33] an three trials did not provide this information [30],[36],[59]. |
|
What types of interventions were included |
All of the trials used chondroitin sulfate. Twenty seven trials used only chondroitin sulfate: [14], [15],[16],[17],[18],[20],[21],[22],[23],[24],[26],[27], [28],[31],[37],[41],[46],[48],[50],[54],[55],[56],[58], [61],[63],[65],[66], eleven trials used chondroitin sulfate and glucosamine [30],[34],[39],[42],[43],[44],[45],[51], [52],[53],[57], five trials used chondroitin sulfate, glucosamine and other components [29],[38],[59],[60], [62], four trials used chondroitin sulfate versus nonsteroidal anti-inflammatory drugs [18],[25],[35],[40] and three trials used chondroitin and did not inform the comparator (no placebo) [33],[36],[64]. Twenty trials used a daily dose of chondroitin sulfate greater than 1000 mg (between 1000 mg and 2000 mg) [16],[17],[18],[19],[21],[25],[28],[31],[36],[37],[48], [50],[51],[53],[56],[57],[59],[63],[64],[66], nineteen trials used a daily dose of chondroitin sulfate lesser than 1000 mg (between 60 mg and 800 mg) [20],[22],[23], [24],[26],[29],[34],[35],[39],[40],[41],[46],[54],[55], [58],[60],[61],[62],[65], seven trials used a dose scheme with more than one dose of chondroitin sulfate [27],[30],[33],[42],[43],[44],[45] and four trials did not provide this data [14],[15],[38],[52]. Forty seven trials used oral administration of chondroitin sulfate [16],[17],[18],[19],[20],[21],[22],[23],[24],[25], [26],[27],[28],[29],[30],[31],[33],[34],[35],[36],[37], [39],[40],[41],[42],[43],[44],[45],[46],[48],[50],[51], Nine trials reported that patients received coadjuvant therapy with acetaminophen [17],[19],[20],[26],[27],[28], [48],[56],[66]. Fourteen trials used ibuprofen, diclofenac or other nonsteroidal anti-inflammatory drugs [14],[25],[35],[36],[38],[40],[42],[43],[44],[52],[59],[60], The treatment mean time of duration was 7 months, with a minimum of 1 month and a maximum of 2 years. Eight trials did not report this data [25],[26],[27], [29],[35],[36],[40],[56]. Thirty-seven trials compared against placebo: [14],[15], [16],[17],[18],[20],[21],[22],[23],[24],[26],[27],[28], [29],[30],[31],[34],[37],[38],[39],[41],[46],[48],[50], [51],[52],[54],[55],[56],[58],[59],[60],[61],[62],[63], [65],[66]. Ten trials compared against nonsteroidal anti-inflammatory drugs [19],[25],[35],[40],[42],[43],[44], [45],[53],[57]. Three trials did not provide this information (no placebo) [33],[36],[64]. |
|
What types of outcomes |
Among the outcomes measured by the systematic reviews were pain in the visual analogue scale (VAS) from 0 to 10 or from 0 to 100 mm, the Western Ontario and McMaster Universities Total Arthritis Index (WOMAC), WOMAC stiffness, Physical Function from 0 to 100 (adapted WOMAC scale), Lequesne Index, reduction of joint space in the radiography, adverse effects, total knee arthroplasty requirement, among others. |
Summary of findings
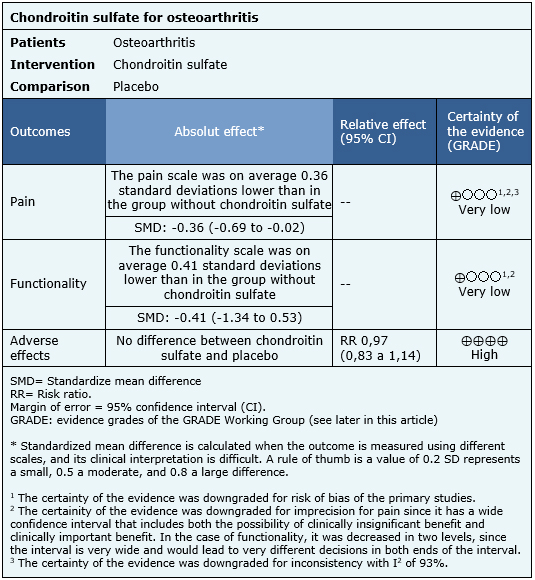
The effects of chondroitin sulfate are based on 18 randomized trials that, in total, included 2188 patients. The rest of the trials did not report the outcomes of interest, or the identified reviews do not provide data suitable for meta-analysis. Eighteen trials [14],[16],[17],[18],[21],[22],[23],[24],[27],[31],[32],[41],[46],[50],[56],[61],[63],[66] measured pain using the visual analogue scale (2188 patients). Only two trials [24],[26] evaluated functionality using the WOMAC scale (403 patients). Adverse effects were obtained directly from one of the systematic reviews identified [13], since it was not possible to extract more information from the other reviews. The summary of findings is as follows:
- It is not clear whether chondroitin sulfate decreases pain in osteoarthritis because the certainty of the evidence is very low.
- It is not clear whether chondroitin sulfate improves functionality in osteoarthritis because the certainty of the evidence is very low.
- Chondroitin sulfate has no adverse effects or they are minimal. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
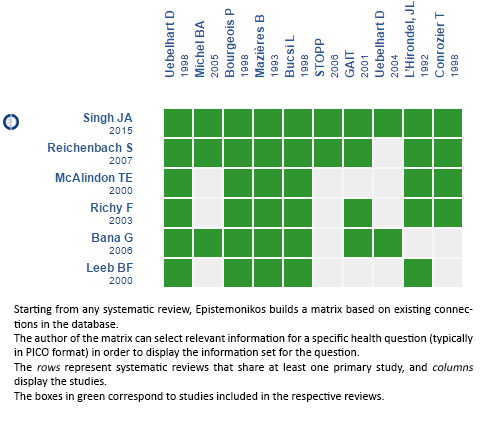
Follow the link to access the interactive version: Chondroitin sulfate for osteoarthritis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

