Epistemonikos summaries
← vista completaPublished on April 18, 2017 | http://doi.org/10.5867/medwave.2017.6931
Is dexamethasone as effective as other corticosteroids for acute asthma exacerbation in children?
¿Es la dexametasona tan efectiva como otros corticoides durante una exacerbación asmática en niños?
Abstract
Dexamethasone has been proposed as an alternative in the treatment of acute asthma exacerbation in children. It allows shortening the duration of treatment, reducing costs and adverse effects. However, it is not clear whether its efficacy is similar to the traditional steroid regimen. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple information sources. We identified six systematic reviews including 10 randomized trials. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded dexamethasone has probably fewer adverse effects than others corticosteroids, and might be equally effective in reducing hospitalizations and revisits.
Problem
Systemic corticosteroids, typically oral prednisone, constitute the cornerstone in the treatment of asthmatic exacerbation in children. However, there is concern about their adverse effects in both the short- and long-term. Dexamethasone allows administration for a shorter period of time, which would reduce adverse effects and costs. It is not clear, however, whether its efficacy is similar.
Methods
We used Epistemonikos database, which is maintained by screening multiple information sources, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] including 10 primary studies relevant to the question of interest, reported in 13 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19]. All of them correspond to randomized controlled trials. Two trials [15],[16] were not considered for this summary since they evaluated adult population. |
|
What types of patients were included |
Regarding the definition of asthma, the different trials used different inclusion criteria: Three trials [7],[8],[19] required a history of clinical episode of wheezing, two trials [12],[17] history of two or more episodes of wheezing, three trials [11],[13],[14] used as inclusion criteria "history of previous asthma". Regarding to age, eight trials [7],[8],[11],[12],[13],[14],[17],[19] focused on pediatric population; six trials with an age range between 2 to 18 years [7],[8],[12],[14],[17],[19], one trial [11] between 1.5 to 7 years and one [13] between 0.5 to seven years. The inclusion criteria were mild or moderate acute asthma exacerbation in four trials [7],[11],[13],[14], moderate acute asthma exacerbation in one [19], three trials [8],[12],[17] excluded patients with severity factors, either life-threatening exacerbation [8], need of intubation [17], or previous history of severe acute asthma exacerbation/need of intubation [12]. Five trials excluded patients who had received corticosteroids in the last four weeks [8],[11],[12],[14], [17], two trials excluded children who received corticosteroids in the last two weeks [7],[13] and in one trial [19] this information could not be obtained from any systematic review. Six trials [7],[8],[11],[12],[14],[17] excluded patients with some chronic comorbidity, one trial [13] did not exclude for this reason, and this information could not be obtained from any systematic review for one trial [19] Other exclusion criteria were exposure to tuberculosis [8], [11], chickenpox [7],[8],[11],[12] and infection by respiratory syncytial virus [8],[13]. One trial [11] excluded patients who presented two episodes of vomiting during emergency consultation after administration of dexamethasone. |
|
What types of interventions were included |
Regarding the intervention group: All of the trials used dexamethasone. It was administered orally in four trials [7],[8],[12],[17], intramuscular in three [11],[13],[14], and nebulized in one [19]. The dose ranged from 0.3 mg/kg/day to 1.7 mg/kg/day. In five trials the treatment lasted one day [7],[8],[11], [13],[14], in two trials [12],[17] it lasted two days, and we were not able to obtain this information for one trial [19].
Three trials [7],[8],[11] compared against prednisolone and five trials [12],[13],[14],[17],[19] against prednisone. The comparison was administered orally in all of the trials. The dose varied between 1-2 mg/kg/day. The duration of treatment was 3 to 5 days, except for one trial [14] that lasted two days. We could not obtain this information for another trial [19]. |
|
What types of outcomes |
The outcomes were pooled by the systematic reviews as follows:
|
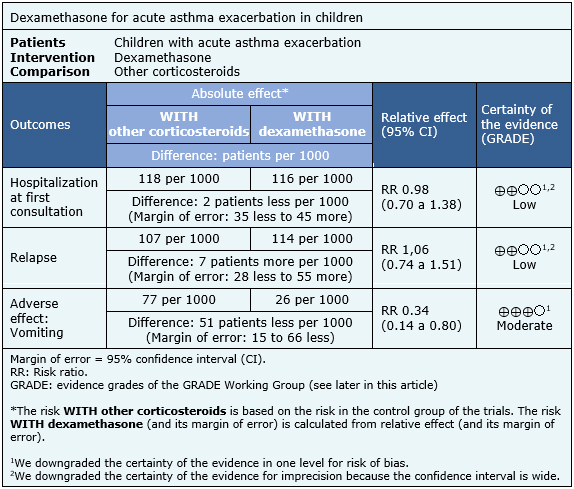
Summary of findings
The information about the effects of dexamethasone compared to others corticosteroids is based on eight randomized trials [7],[8],[11],[12],[13],[14],[17],[19] including 1280 patients.
Three trials [7],[8],[17] reported hospitalization at first consultation (1007 patients), eight trials [7],[8],[11],[12],[13],[14],[17],[19] reported relapse (1280 patients) and five trials [7],[8],[11],[12], [17] (1112 patients) reported adverse effects (vomiting).
The summary of findings is the following:
- Dexamethasone may be equally effective as other corticosteroids in reducing hospitalizations in children with acute asthma exacerbation, but the certainty of the evidence is low.
- Dexamethasone may be equally effective as other corticosteroids in reducing hospitalizations, and revisit to emergency services or unscheduled medical consultations in children with acute asthma exacerbations, but the certainty of the evidence is low.
- Dexamethasone may be equally effective as other corticosteroids in reducing revisit to emergency service or non-scheduled medical consultation in children with acute asthma exacerbation, but the certainty of the evidence is low.
- Dexamethasone, compared to other corticosteroids, probably has less adverse effects (vomiting) in children with acute asthma exacerbation. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
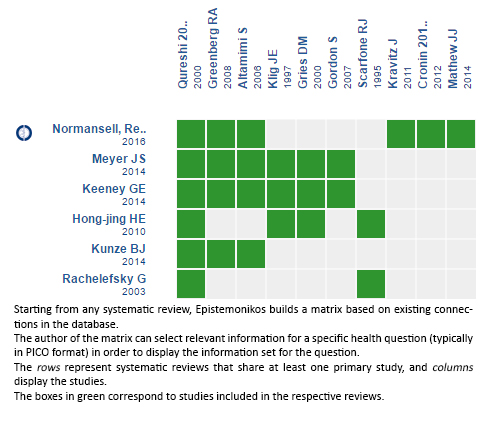
Follow the link to access the interactive version: Dexamethasone versus others corticoids for acute asthma exacerbation
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

