Epistemonikos summaries
← vista completaPublished on May 23, 2017 | http://doi.org/10.5867/medwave.2017.6958
Is it worth adding loperamide to antibiotic treatment of traveler’s diarrhea?
¿Vale la pena agregar loperamida al tratamiento antibiótico de la diarrea del viajero?
Abstract
Travelers' diarrhea is a frequent condition, especially in those traveling to high-risk areas. Although antibiotic treatment reduces the duration of diarrhea, it has been suggested adding loperamide could further reduce the symptoms. To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified two systematic reviews including 28 studies overall, of which 15 were randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reanalysed data of primary studies and generated a summary of findings table using the GRADE approach. We concluded adding loperamide to antibiotic treatment might accelerate resolution of symptoms in traveler’s diarrhea with minimal or no adverse effects.
Problem
Travelers' diarrhea is a common condition affecting approximately 20-60% of those traveling to high-risk areas, usually low-income regions. Although it is not generally associated to serious complications, it entails morbidity, disability, and costs.
Antibiotic treatment is considered the standard treatment, but despite its use a substantial number of people persist with symptoms at 48 hours.
Loperamide is an antimotility agent that acts at the level of intestinal opioid receptors, reducing peristalsis. It has been suggested that adding it to antibiotic treatment could reduce the time of symptoms, as well as the duration of antimicrobial treatment, which would result in lower costs, adverse effects and antibiotic resistance.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies if data are suitable, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found two systematic reviews [1],[2] including 28 studies relevant for the question of interest [3],[4],[5], [6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17], [18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28], [29],[30], including 15 randomized controlled trials [3], |
|
What types of patients were included* |
Four trials included military personnel sent abroad for military operations [12],[13],[15],[16].The rest of the trials included regular people traveling. The destinations were: |
|
What types of interventions were included* |
Loperamide was used in all trials, with a maximum dose of 16 mg per day. Only two trials compared antibiotic treatment versus loperamide alone [4],[6], and one trial compared loperamide versus education, with co-interventions in both groups [16]. In the rest of the trials, antibiotic monotherapy was compaired to loperamide plus antibiotic, using the following antibiotic schemes:
In some trials other cointerventions were used, such as the use of zaldaride [11],[14] or bismuth [16],[17]. |
|
What types of outcomes |
The outcomes studied were:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
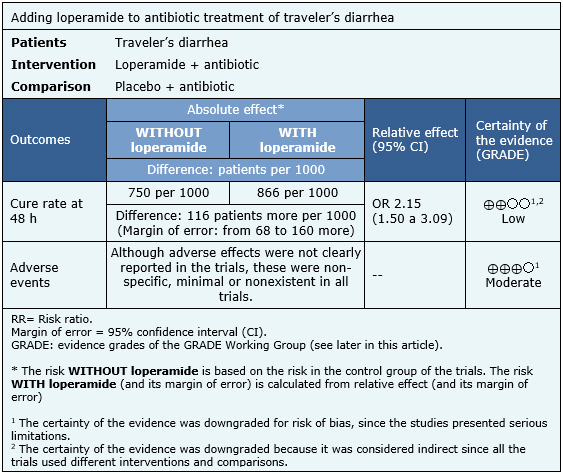
Information on the effects of adding loperamide to the antibiotic treatment of traveler's diarrhea is based on 15 randomized trials involving 2527 patients. It was not possible to conduct meta-analysis from the data extracted from the different reviews, so the meta-analysis provided by an individual review was used [2].
The summary of finding is as follows:
- Adding loperamide to antibiotic treatment might accelerate resolution of traveler’s diarrhea, but the certainty of the evidence is low.
- There are probably minimal or no adverse effects of loperamide when used in the treatment of traveler's diarrhea.The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
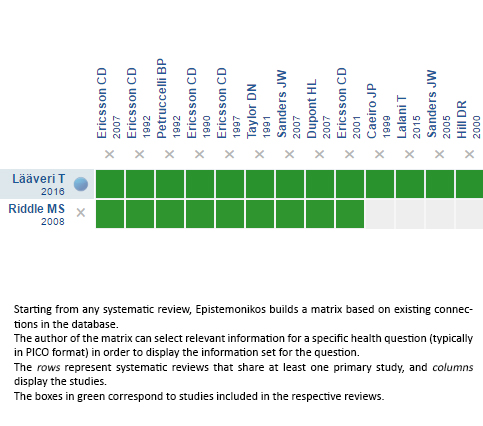
Follow the link to access the interactive version: Loperamide for traveler's diarrhea
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

