Resúmenes Epistemonikos
← vista completaPublicado el 6 de junio de 2017 | http://doi.org/10.5867/medwave.2017.6965
¿Cuál es la efectividad de los corticoides sistémicos en la laringitis aguda obstructiva en niños?
What is the effectiveness of systemic corticosteroids in children with croup?
Abstract
Systemic corticosteroids constitute standard treatment in children with acute obstructive laryngitis (croup). However, there is some uncertainty in relation with the magnitude of the benefits and risks associated with their use. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews including 25 randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reananalysed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded the use of systemic corticosteroids increases the number of patients with clinical improvement at 12 hours and reduces the risk of readmission.
Problem
Acute obstructive laryngitis is one of the most frequent respiratory diseases in childhood, predominantly in children between 6 months and 6 years, with a higher incidence in late autumn and early winter [1],[2]. They are usually mild and self-limiting, although occasionally they can cause severe respiratory obstruction. Systemic corticosteroids reduce laryngeal edema by reducing the local inflammatory reaction, contracting lymphoid inflammation and impairing capillary permeability [1],[3]. Due to their potent anti-inflammatory effect, they have been one of the pillars of treatment in patients with croup. However, there is some degree of uncertainty regarding the magnitude of the benefits.
On the other hand, although the use of corticosteroids in high doses, or for long time, is associated to multiple adverse effects, the use of a single dose in acute obstructive laryngitis would constitute a safe therapy [4].
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [4],[5],[6],[7],[8],[9] including twenty-five randomized controlled trials [10], [11],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21], [22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32], [33],[34] evaluating the use of corticosteroids compared to placebo. |
|
What types of patients were included* |
Eight trials included patients who consulted in an emergency department [12],[15],[16],[19],[21],[22],[25], [31] and sixteen included patients in the hospital setting [10],[11],[13],[14],[17],[18],[20],[23],[24],[26],[28], [29],[30],[33],[34]. One trial considered a group evaluated in emergency services that was then admitted to the hospital [32].The age range ranged from 3 months to 12 years. Three trials included patients with mild laryngitis [12], [16],[31], three with moderate laryngitis [21],[22],[25] and one mild to moderate [15]. Eighteen trials did not classify the severity of laryngitis [10],[11],[13],[14],[17], [18],[19],[20],[23],[24],[26],[27],[28],[29],[30],[32], |
|
What types of interventions were included* |
Seventeen trials considered only the use of systemic corticosteroids (oral, nasogastric, intravenous, subcutaneous and intramuscular) [12],[13],[14],[17],[18], The corticosteroids used were prednisone, dexamethasone, methylprednisolone, prednisolone and budesonide. The dosages used had great variation between the different trials. From the eighteen trials that considered the use of systemic corticosteroids, fourteen used dexamethasone in different single doses (0.6 mg/kg [12], [16],[17],[22],[25],[32],[33], 0.15 mg/kg [31], 0.3 mg/kg [19], 0.4 mg/kg [29], 0.5 mg/kg [24], between 4 and 12 mg total [20],[23],[28]). Two trials used 4 mg/kg methylprednisolone once or twice [14],[26] (equivalent to 0.8 mg/kg dexamethasone dose). Two trials used prednisolone, one at a dose of 2 mg/kg up to 24 hours post-extubation [13] (equivalent to 0.3 mg/kg dexamethasone), and another 2.5 mg at 6 hours without specifying time of treatment [18]. One trial used prednisone 2.5 to 5 mg three doses in one day (equivalent to 0.4 to 0.8 mg dexamethasone dose) [34] and one trial used dexamethasone used 0.04 mg/kg/day in 4 doses [30]. The rest of the trials used the inhalation route with budesonide from 1 to 4 mg per dose up to 4 doses, and nebulized dexamethasone from 10 to 20 mg. |
|
What types of outcomes |
The trials measured multiple outcomes, however, the different systematic reviews grouped them as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
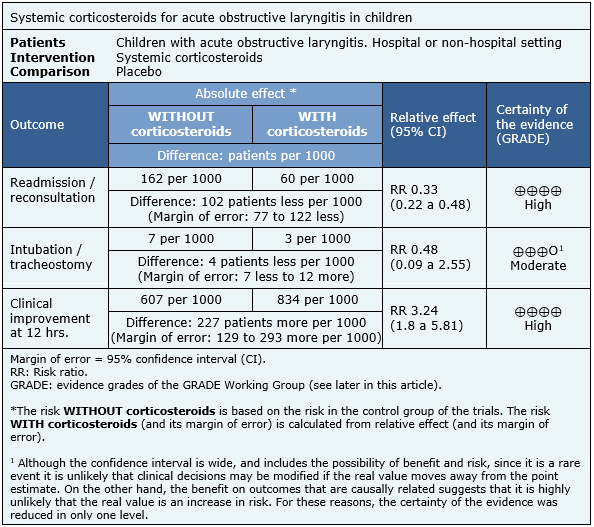
The information on the effects of systemic corticosteroids in children with acute obstructive laryngitis is based on 13 randomized trials. The rest of the trials were not analyzed because they did not consider systemic steroids, or did not report the outcomes of interest. Six trials measured the outcome readmissions/reconsultations [12],[16],[22],[25],[28],[31], six endotracheal intubation/tracheostomy [19], [20],[22],[23],[28],[29] and five clinical improvement at 12 hours [14],[23],[26],[29],[33]. The summary of findings is as follows:
- The use of systemic corticosteroids in children with acute obstructive laryngitis reduces the risk of readmission. The certainty of the evidence is high.
- The use of systemic corticosteroids in children with acute obstructive laryngitis probably reduces the probability of requiring intubation or tracheostomy. The certainty of the evidence is moderate.
- The use of systemic corticosteroids in children with acute obstructive laryngitis increases the number of patients with clinical improvement at 12 hours. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
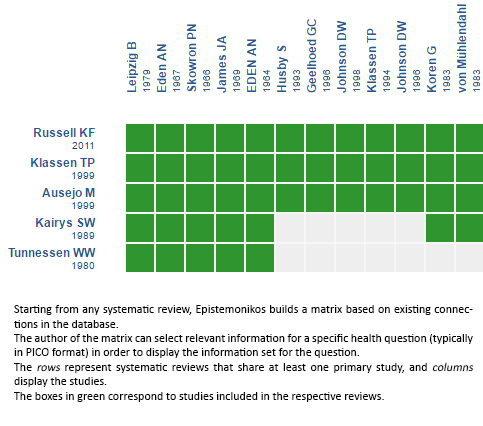
Follow the link to access the interactive version: Systemic corticosteroids versus placebo for croup
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

