Resúmenes Epistemonikos
← vista completaPublicado el 9 de agosto de 2017 | http://doi.org/10.5867/medwave.2017.07.7010
¿Es el cannabidiol un tratamiento efectivo para la esquizofrenia?
Is cannabidiol an effective treatment for schizophrenia?
Abstract
Cannabidiol has recently been proposed as an antipsychotic for schizophrenia. However, its clinical use and safety is controversial. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews incorporating four primary studies overall, including two randomized trials. We extracted data from the systematic reviews, reanalyzed data from primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabidiol probably does not improve symptoms in schizophrenia and leads to frequent side effects.
Problem
Research has shown the endocannabinoid system plays an important role in the pathophysiology of schizophrenia. Several studies have concluded the use of cannabis is a risk factor for development and early onset of the illness. Moreover, tetrahydrocannabinol has been associated to psychotic symptoms typical of schizophrenia like hallucinations, delusions, depersonalization, and others [1],[3],[15],[16].
Nonetheless, cannabidiol might exert an antipsychotic effect opposing tetrahydrocannabinol in the brain and having an inverse relation with the positive symptoms of schizophrenia. Its mechanism of action is not clear, but it is suggested it would involve the cannabinoid receptors CB1/CB2 which inhibit anandamide reuptake, increasing endocannabinoid levels.
However, its efficacy and safety for the treatment of schizophrenia are not certain.
Methods
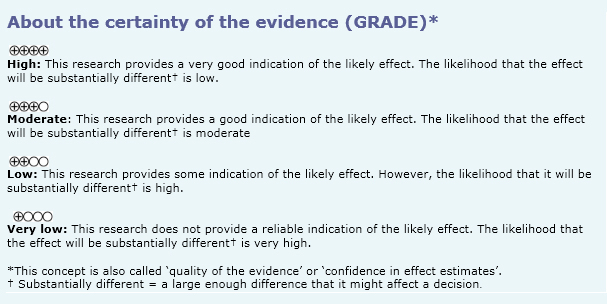
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6], including four primary studies reported in seven references [7],[8],[9],[10],[11],[12],[13], including two randomized trials [7],[12]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
All the trials included patients with the diagnosis of schizophrenia, but only one specified the type, including patients with acute paranoid schizophrenia or schizophreniform disorder. |
|
What types of interventions were included* |
One trial used 600 mg of cannabidiol orally [12] and one trial used 300 mg or 600 mg of cannabidiol orally [7]. It could not be retrieved information related to the baseline treatment of patients. Both trials compared against placebo. |
|
What types of outcomes |
The different systematic reviews used the following psychiatric scales as outcome measure of the improvement of psychotic symptoms:
In addition, one trial evaluated the cognitive deficit through the SCWT scale (Stroop Color Word Test) Other outcomes evaluated were the presence of side effects associated to the traditional treatment of schizophrenia like extrapyramidal symptoms, levels of prolactine and weight gain. One trial lasted four weeks [12]. No information about the duration of the second trial could be retrieved. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
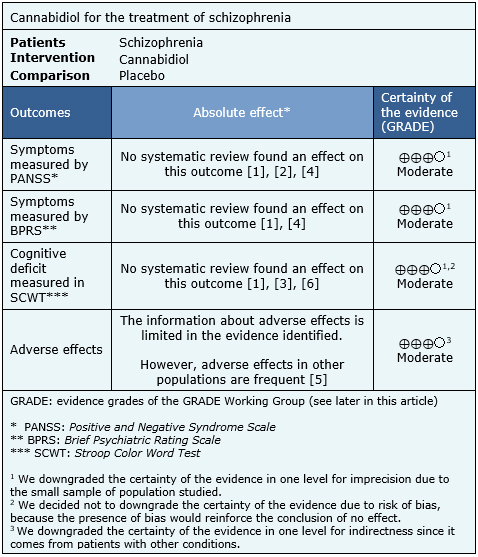
Summary of findings
The information about the effects of cannabidiol in schizophrenia is based on two randomized trials that include 57 patients [7],[12].
Both trials measured the improvement of symptoms through the PANSS and BPRS scale. One trial measured the cognitive deficit through the SCWT. None of the reviews identified were able to extract data in a way that could be incorporated in a meta-analysis, so the information presented below corresponds to a narrative synthesis of the information obtained from the reviews.
The summary of findings is the following:
- Cannabidiol probably does not decrease symptoms in schizophrenia measured by the PANSS scale. The certainty of the evidence is moderate.
- Cannabidiol probably does not decrease symptoms in schizophrenia measured by the BPRS scale The certainty of the evidence is moderate.
- Cannabidiol probably does not improve cognitive deficit of schizophrenia measured in SCWT. The certainty of the evidence is moderate.
- Cannabinoids probably lead to frequent adverse effects in patients with schizophrenia. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
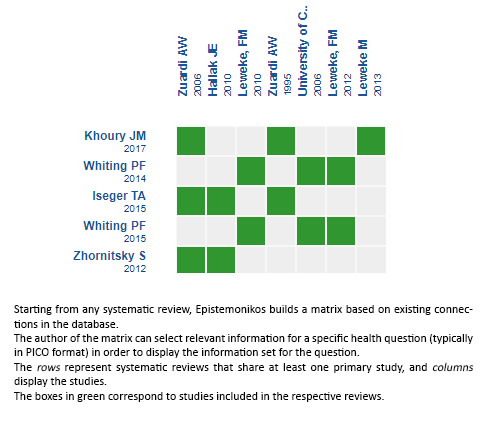
Follow the link to access the interactive version: Cannabidiol versus placebo for the treatment of schizophrenia
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

