Epistemonikos summaries
← vista completaPublished on August 31, 2017 | http://doi.org/10.5867/medwave.2017.07.7020
Do textured breast implants decrease the rate of capsular contracture compared to smooth implants?
¿Implantes mamarios texturizados o lisos para disminuir la tasa de contractura capsular?
Abstract
The use of breast implants for aesthetic and reconstructive purposes has become one of the most common procedures performed by plastic surgeons. Several breast implants models exist. They differ in their size, filling, shape and characteristic of the shell, which can be smooth or textured. Capsular contracture is one of the main complications of breast implants. It has been suggested that the use of textured implants could reduce the incidence of capsular contracture. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified 15 studies overall, of which 13 were randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded the use of textured breast implants probably decreases the risk of capsular contracture, however, they might be associated to an increased risk of anaplastic large cell lymphoma.
Problem
The use of breast implants for aesthetic and reconstructive purposes has become one of the main performed procedures by plastic surgeons. According to statistics presented by the International Society of Aesthetic Plastic Surgeons, 1.3 million breast implants were implanted during the year 2015 [1]. Capsular contracture, which is a contractile deformation of the periprosthetic capsule, is one of the main complications of breast implants. Its estimated incidence is 7.6% per implant [2] and is responsible of up to 30% of revision surgeries [3]. Although its etiology is not fully elucidated, it appears to be a multifactorial process [4]. Several risk factors have been implicated, such as the presence of postoperative hematoma, the implant site, the access incision and the type of surgery (aesthetic versus reconstructive). Implant risk factors such as the type of filling (silicone versus saline) and the shell texture (smooth versus textured) have also been described [2],[5]. The main studies evaluating the role of breast implant texture have not been conclusive, [6],[7] so there is still controversy.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [8],[9],[10],[11],[12] which together include a total of 15 primary studies reported in 18 references [6],[7],[13],[14],[15],[16], |
|
What types of patients were included* |
All trials included patients who received breast implants with a silicone elastomer envelope. Eight trials included patients with breast augmentation [6],[7],[14],[15],[16],[18],[20],[27], three trials with previous mastectomy [21],[23],[25], one with both etiologies [17], and one trial with no clear etiology [26]. Seven trials included silicone filled implants [14],[18],[20],[21],[25],[26],[27] while four included patients with saline implants alone [7],[15],[16],[23]. One trial used both implants [17]. The implant site also differed between trials. In nine, the implant placement site was subcutaneous [6],[7],[15],[18], [20],[26],[27] whereas in one it was retromuscular [14], and in three it was not specified [21],[23],[25]. Two trials included patients with periareolar incisions [15], [16], two with inframammary incisions [6], and [14] three with transaxillary approach [17],[26],[27]. In three trials the incision previously made for the mastectomy was utilized [21],[23],[25] and in three others no information was provided regarding the approach site [7],[18],[20]. In five trials the follow-up was up to one year [14],[15], [17],[18],[23] and in four up to 2 years [16],[25],[26] , [27]. In the four remaining trials, follow-up was 3, 5, 7.5 and 10 years respectively [6],[18][20],[21]. In only four trials, the follow-up rate was 100% [7],[23],[25],[26] |
|
What types of interventions were included* |
In the 13 evaluated trials smooth implants were compared to textured implants. In five, the breast side was randomized to receive each type of implant [6],[7],[15], [16],[20], whereas in one trial the patient was randomized [18]. No further information could be obtained from the other trials. |
|
What types of outcomes |
The following outcomes were measured:
The median follow-up time, considering all the trials was 2 years (range 1 to 10 years). |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
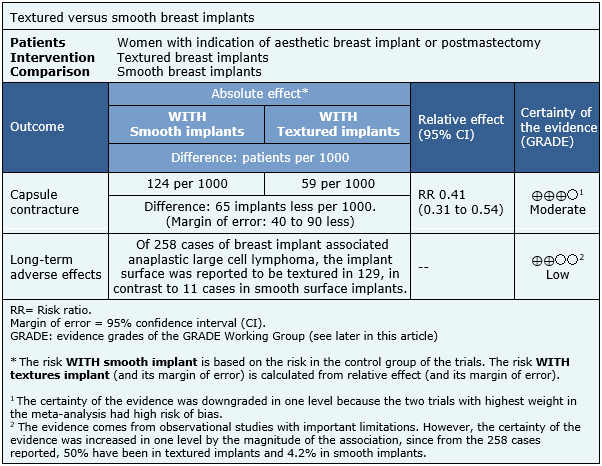
Summary of findings
The effect of textured implants on the risk of capsular contracture is based on 13 randomized trials involving a total of 2302 breast implants (1255 smooth and 1047 textured) [6],[7],[14],[15],[16],[17],[18],[20],[21],[23],[25],[26],[27]. Data on the risk of long-term adverse effects comes from case reports [28],[29].
The summary of findings is as follows:
- The use of textured breast implants probably reduces the risk of capsular contracture. The certainty of the evidence is moderate.
- The use of textured breast implants might be associated to an increased risk of anaplastic large cell lymphoma, but the certainty if this evidence is low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
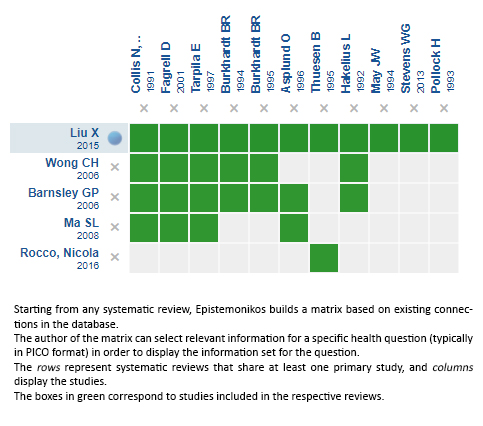
Follow the link to access the interactive version: Textured versus smooth implant in patients with breast augmentation and reconstruction
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

