Resúmenes Epistemonikos
← vista completaPublicado el 26 de diciembre de 2017 | http://doi.org/10.5867/medwave.2017.09.7111
¿Son útiles los corticoides sistémicos en el manejo de la faringitis aguda?
Are systemic corticosteroids useful for the management of acute pharyngitis?
Abstract
INTRODUCTION Pain associated to acute pharyngitis is a frequent cause of consultation. Usual care includes non-steroidal anti-inflammatories and antibiotics in selected cases, but pain relief is not always quick enough. The use of corticosteroids has been proposed as a therapeutic alternative, but its actual efficacy is matter of debate.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified eight systematic reviews including 11 studies overall, of which 10 were randomized trials. We concluded a short course of systemic corticosteroids reduces pain related to acute pharyngitis, without increasing the risk of adverse effects.
Problem
Acute pharyngitis is a frequent condition. Viral etiology predominates, but a small percentage of bacterial etiology exists, among which group A beta-hemolytic Streptococcus stands out.
Acute pharyngitis related pain is a frequent reason for consultation in primary care and emergency departments. Usual care with non-steroidal anti-inflammatories and antibiotics in selected cases does not relieve pain completely and timely in all cases.
Corticosteroids have been proposed for the management of pain related to acute pharyngitis based on their potent anti-inflammatory effect.
The present summary aims to determine if corticosteroids constitute an effective and safe intervention for the management of acute pharyngitis.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified eight systematic reviews [1],[2],[3],[4],[5],[6],[7],[8], including 11 primary studies [9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19], of which 10 corresponded to randomized trials [9],[10],[12],[13],[14],[15],[16],[17],[18],[19]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
All of the trials considered patients presenting with acute pharyngitis. Six trials only included adult patients [9],[12],[13],[14],[18],[19], three only pediatric patients [10],[15],[17] and one did not differentiate [16]. Average age was 24 years, including patients from four years and older. Detection of group A beta-hemolytic Streptococcus was an inclusion criterion in one trial [15]. Two trials did not report detection of this pathogen [16],[18]. The remaining trials reported detection in 14.9 to 57.5% of patients [9],[10],[12],[13],[14],[17],[19]. Among exclusion criteria the following stand out: previous use of antibiotics in two trials [10],[12], pregnancy in nine trials [10],[12],[13],[14],[15],[16],[17],[18] and diabetes mellitus in nine trials [9],[10],[12],[13],[14],[16],[18],[19]. All trials excluded immunocompromised patients, suspected peritonsillar or retropharyngeal abscess and previous use of corticosteroids. Three trials were conducted in primary care [10],[12],[13], while the remaining trials were conducted in emergency departments [9],[14],[15],[16],[17],[18],[19]. |
|
What types of interventions were included* |
All of the trials compared systemic corticosteroids against placebo or no treatment. Most trials used a single 8-10 mg or 0.6 mg/kg dose of dexamethasone [9],[10],[12],[15],[16],[17],[18],[19]. One trial used prednisone [13] and one used betamethasone [14], both as single dose. Route of administration was oral in seven trials [9],[10],[12],[13],[15],[17],[19] and intramuscular in three [14],[16],[18]. In six trials, all patients received antibiotics [9],[14],[15],[16],[18],[19]. In four trials, the indication of antibiotics was at physician discretion or when group A beta-hemolytic Streptococcus was detected [10],[12],[13],[17]. In four trials, all patients received analgesia [9],[15],[18],[19] and in the remaining trials analgesia was at physician discretion [10],[12],[13],[14],[16],[17]. |
|
What types of outcomes |
Systematic reviews grouped outcomes as follows: resolution of pain at 24 or 48 hours, time to onset of pain relief, time to complete resolution of pain, pain intensity (visual analog scale) at 24 hours, symptoms recurrence, antibiotic prescription, work and school absentism, and adverse effects. Mean follow up time in trials was 18 days, with a range from three days to two months. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information about the effects of corticosteroids for acute pharyngitis is based on ten randomized trials including 966 patients [9],[10],[12],[13],[14],[15],[16],[17],[18],[19]. Five trials reported resolution of pain at 24 hours [12],[13],[15],[18],[19]. The remaining trials reported pain in other ways (see other considerations for decision-making section). Eight trials reported adverse effects [10],[12],[14],[15],[16],[17],[18],[19]. The summary of findings is the following:
- Systemic corticosteroids increase the number of patients with resolution of pain at 24 hours. The certainty of the evidence is high.
- Systemic corticosteroids do not increase adverse effects or these are minimal. The certainty of the evidence is high.
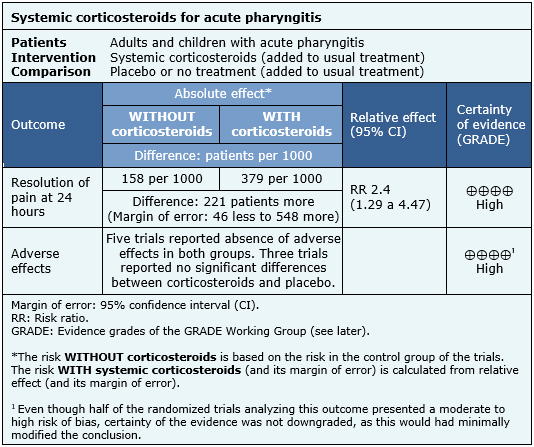
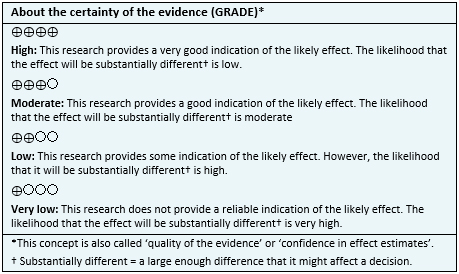
| Following the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
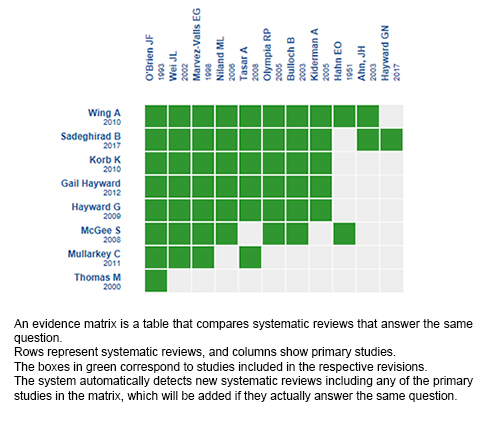
Follow the link to access the interactive version: Corticosteroids versus placebo or no treatment for acute pharyngitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

