Resúmenes Epistemonikos
← vista completaPublicado el 28 de diciembre de 2017 | http://doi.org/10.5867/medwave.2017.09.7119
¿Son efectivos los cannabinoides para el manejo de náuseas y vómitos inducidos por quimioterapia?
Are cannabinoids effective for the management of chemotherapy induced nausea and vomiting?
Abstract
INTRODUCTION Nausea and vomiting are common side effects in cancer patients treated with chemotherapy. Proper control of these symptoms might improve quality of life in these patients. Addition of cannabinoids to standard antiemetic treatment has been proposed in order to improve control of these symptoms.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified 16 systematic reviews that include 61 primary studies. Out of these, four were randomized trials that answered our question. At present, given that the certainty of the evidence is very low, it is unclear whether the addition of cannabinoids to standard antiemetic regimes benefits patients with chemotherapy induced nausea and vomiting. Cannabinoids probably increase adverse effects substantively.
Problem
Acute and delayed nausea and vomiting are commonly associated to chemotherapy treatments. These are common side effects usually known and feared by oncological patients [1],[2]. In recent years, several new drugs have been incorporated into treatment regimes in order to prevent and manage emesis. Despite this, some patients remain unresponsive to standard management [3]. The use of cannabinoids (delta-9 tetrahydrocannabinol (THC) or its derivatives) as antiemetic has been postulated mainly based on its effects upon CB1-endocannabinoid receptors located at the central nervous system and their action over the emetic center of the brain, similar to the 5-HT3 serotonin receptors [4],[5]. However, from a clinical point of view, the role of cannabinoids upon chemotherapy-induced emesis is still controversial.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 16 systematic reviews [6],[7],[8],[9],[10], This summary analyzed four trials, reported across eight references [22],[23],[24],[25],[26],[27],[28],[29], that compared the effect of cannabinoids against placebo in patients under an antiemetic regime, reporting the control of nausea and vomiting during the intervention period, which is the clinically relevant question for the authors. |
|
What types of patients were included* |
All selected trials included male and female adults. The trials also included elderly patients, up to 81 years old. Patients included in these trials had solid [22],[25], and solid or hematological tumors [23],[24]. The trials included chemotherapy regimes with a high to moderate [22], only moderate [23],[25], and not classifiable risk of emesis [24] (the latter included several chemotherapies with a high risk of emesis, but also some with low to minimum risk). Three out of four trials reported previous use of cannabinoids at any time, one of these trials included only patients with no previous exposure [24], and 2 trials included patients with or without previous exposure [22],[25]. |
|
What types of interventions were included* |
Studied cannabinoids were dronabinol (a synthetic THC derivative) with a maximum dose of 10 mg/day [22], 15 mg/day [23] or 40 mg/day [24] and nabiximol (a THC and cannabidiol extract) up to 8 sprays over a 4-hour period every 24 hours [25]. Regarding the associated antiemetic regime, two trials used corticosteroids combined with 5HT-3 antagonists [22],[23]; one trial used corticosteroids plus 5HT-3 antagonists or metoclopramide [25], and one study used prochlorperazine only [24]. |
|
What types of outcomes |
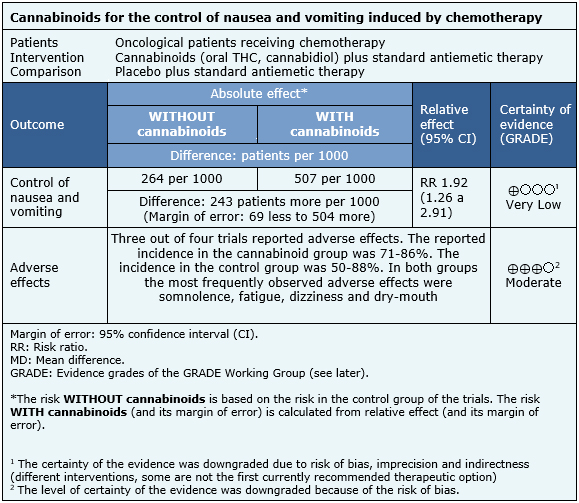
All trials evaluated the control of nausea and vomiting during the study period [22],[23],[24],[25] quantifying partial and complete responses. Three out of four of these trials [22],[24],[25] reported adverse effects. Also, some trials measured the tolerability of the intervention (measured as dropout from treatment due to adverse effects), the impact of nausea and vomiting upon quality of life, its frequency, duration and severity [22],[24],[25], percentage of patients and physicians satisfied with the intervention [25], ECOG score and quality of life [22]. Three out of four trials had a 5-day follow up [22],[23],[25] and one had a 6-day follow up [24]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information obtained about the effects of cannabinoids on nausea and vomiting induced by chemotherapy is based on four randomized trials that involved a total of 176 patients [22],[23],[24],[25]. All four trials measured control of nausea and vomiting, while three measured the occurrence of adverse events [22],[24],[25].
The summary of findings is the following:
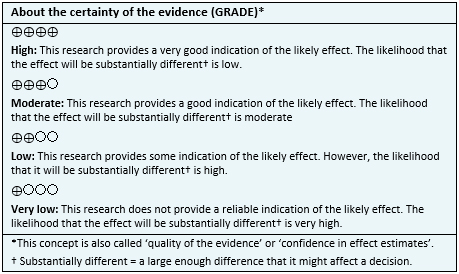
- It is unclear whether adding cannabinoids to standard antiemetic regimes improves the control of nausea and vomiting induced by chemotherapy, because certainty of the evidence is very low.
- The use of cannabinoids probably increases the occurrence of adverse effects in these patients. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
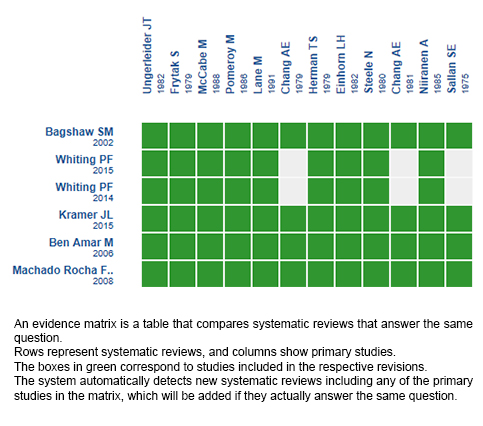
Follow the link to access the interactive version: Cannabinoids for chemotherapy induced nausea and vomiting
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

