Resúmenes Epistemonikos
← vista completaPublicado el 29 de diciembre de 2017 | http://doi.org/10.5867/medwave.2017.09.7130
¿Son los cannabinoides una opción para el síndrome anorexia-caquexia en pacientes con cáncer avanzado?
Are cannabinoids an alternative for cachexia-anorexia syndrome in patients with advanced cancer?
Abstract
INTRODUCTION Cachexia and anorexia are among the most frequent symptoms in patients with cancer. Cannabinoids have been used in patients with advanced cancer; however, their role is still controversial.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified ten systematic reviews including three studies overall, of which two were randomized trials. We concluded it is not clear whether cannabinoids have any positive effect on increasing weight because the certainty of the evidence is very low. They might not have any effect on appetite, and are probably associated to frequent adverse effects.
Problem
The anorexia-cachexia syndrome corresponds to an hypercatabolic state characterized by an absence of desire to eat and an involuntary weight loss associated with a loss of muscle mass. This phenomenon is observed in up to 80% of patients with cancer and in variable percentages in patients with other non-oncological diseases [1].
In oncological patients, anorexia is explained by a variety of causes (e.g. dysphagia, dry mouth, nausea, depression), which determine a decrease in intake and in the availability of nutrients, and as a consequence, weight loss. In patients with isolated anorexia, the increase of the intake and the availability of nutrients can revert weight loss. Although anorexia by itself does not explain the deterioration observed in cancer patients, it is a factor that contributes to weight loss and is considered a relevant parameter in the quality of life [2]. Cachexia corresponds to a multifactorial syndrome characterized by loss of skeletal muscle mass, with or without loss of fat mass, which leads to progressive functional deterioration [3]. It is among the most common symptoms in cancer patients, with a prevalence of 50% at the time of diagnosis and 80% at the end-of-life stage. It is associated with a decrease in quality of life and survival (it can be the direct cause of 20% of deaths related to this disease) and to other symptoms such as anorexia (in up to 90% of cancer patients), fatigue, asthenia, weakness, early satiety, among others [4]. It is known the pathophysiology of cachexia is multifactorial; for example, tumor growth is associated with profound metabolic and neurochemical alterations, such as the production of a series of proinflammatory cytokines, including TNF-alpha, IL-1 and IL-6, which favour an hypercatabolic state and hamper the use of available nutrients.
Several interventions focused on these particular mechanisms have been proposed, within which we find progestogens, corticoids and cannabinoids [5], the latter would seek to improve appetite through the activation of the CB 1 (central nervous system) and CB 2 (immune cells) receptors of the endocannabidiol system [6], and to decrease weight loss through immunomodulating effect of cytokines [7]. However, it is not clear if this theory actually translates into a clinical effect.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 10 systematic reviews [8],[9],[10],[11],[12],[13],[14],[15],[16],[17] that included three primary studies relevant to the question [18],[19],[20]; two of these correspond to randomized trials [18],[19]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
Both trials included adults (older than 18 years), of both sexes with solid or stage IV hematologic cancer. One trial established inclusion criteria for weight loss in the last 2 to 6 months and also selected by ECOG less than 2 [18]. |
|
What types of interventions were included* |
Both trials evaluated oral delta-9-tetrahydrocannabinol (THC). One trial [18] used oral THC (5 mg / day) and cannabidiol (2 mg / day) for six weeks versus placebo. The other trial [19] used only oral THC in three daily doses (0.1 mg / kg / day) for two weeks versus placebo. |
|
What types of outcomes |
The trials measured multiple outcomes, but the systematic reviews pooled them as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
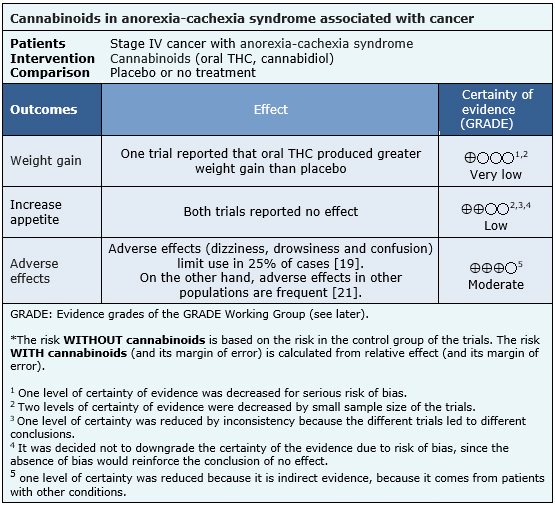
The information about the effects of cannabinoids for anorexia-cachexia syndrome in patients with advanced cancer is based on two randomized trials involving 218 patients.
One trial [18] reported weight gain (164 patients). Both trials [18],[19] reported increased appetite (218 patients).
The summary of findings is as follows:
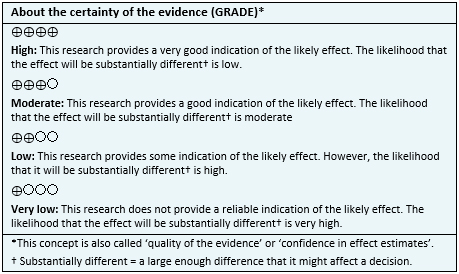
- It is not clear whether the use of cannabinoids leads to weight gain in patients with advanced cancer, because the certainty of the evidence is very low.
- The use of cannabinoids might not increase appetite in patients with advanced cancer. The certainty of the evidence is low.
- The use of cannabinoids is probably associated to frequent adverse effects. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
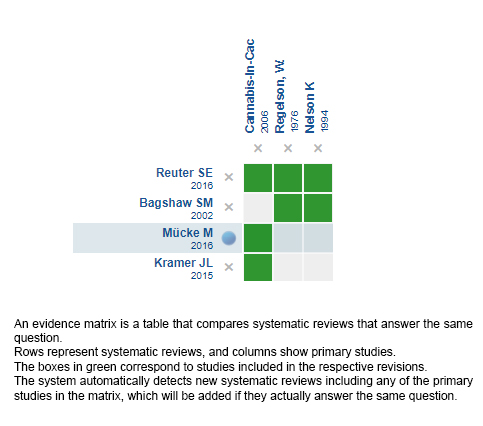
Follow the link to access the interactive version: Cannabinoids versus placebo for cachexia-anorexia syndrome
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

