Epistemonikos summaries
← vista completaPublished on February 13, 2018 | http://doi.org/10.5867/medwave.2018.01.7151
Do cannabinoids constitute a therapeutic alternative for insomnia?
¿Constituyen los cannabinoides una alternativa terapéutica para insomnio?
Abstract
INTRODUCTION It has been suggested that cannabinoids would constitute a therapeutic alternative for patients with insomnia.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified eight systematic reviews including three studies overall, of which two were randomized trials. We concluded it is not clear whether cannabinoids have an effect on insomnia severity or on sleep quality; that they might have no effect on sleep conciliation, sleep awakening or behavior during wakefulness, and are probably associated with frequent adverse effects.
Problem
Insomnia is the most frequent sleep disorder in general population and represents a common reason for consultation. This disorder has a great impact on the waking state, the work capacity and the quality of life of people who suffer from it.
It has been proposed cannabinoids would have an effect on the stimulation of sleep and thus could be an effective treatment for patients with insomnia and other sleep disorders. However, its clinical efficacy and safety are a matter of debate.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
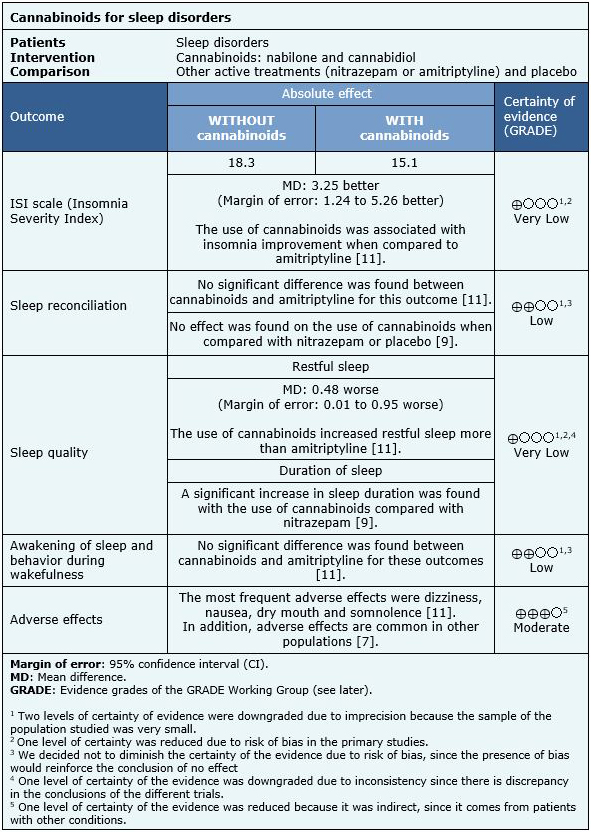
We found eight systematic reviews [1],[2],[3],[4],[5],[6],[7],[8] that included three primary studies reported in six references [9],[10],[11],[12],[13],[14], of which two correspond to randomized trials, reported in five references [9],[11],[12],[13],[14]. This table and the summary in general are based on the randomized trials, since the observational study did not increase the certainty of the existing evidence, nor did provide relevant additional information. |
|
What types of patients were included* |
The first trial [9] included 15 patients with insomnia (diagnostic criterion was not specified). The second trial [11] included 32 patients diagnosed with chronic insomnia, defined as a sleep disturbance according to self-report at least every other night alternately for a minimum of 6 months. Those who fulfilled these criteria were selected within a population of patients with fibromyalgia. In this last trial, a negative urine test for cannabinoids was also required. The average age was 49.5 years and 16% were men. |
|
What types of interventions were included* |
The first trial [9] compared cannabidiol at doses of 40, 80 and 160 mg versus nitrazepam 5 mg and versus placebo. The second trial [11] compared the effect of nabilone between 0.5 to 1 mg versus amitriptyline between 10 to 20 mg. All these drugs were administered orally once a day, before going to sleep. |
|
What types of outcomes |
The first trial [9] measured the duration of sleep and the induction of sleep. The second trial [11] measured the results according to the ISI (Insomnia Severity Index) and LSEQ (Leeds Sleep Evaluation Questionnaire) scales. LSEQ evaluates the reconciliation of sleep, quality of sleep, awakening from sleep and behavior during wakefulness. In addition, the occurrence of adverse effects associated with the use of cannabinoids was evaluated. The follow-up of the first trial [11] was 6 weeks (2 weeks each period separated by 2 weeks), the second trial [9] did not specify the duration of follow-up, only that it was "acute". |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information on the effects of cannabinoids on insomnia is based on two randomized trials [9],[11] that included 47 patients.
The first trial [9] measured the duration of sleep and the induction of sleep, the second trial [11] measured the results according to the ISI (Insomnia Severity Index) and LSEQ (Leeds Sleep Evaluation Questionnaire) scales. LSEQ evaluates the reconciliation of sleep, quality of sleep, awakening from sleep and behavior during wakefulness.
The summary of findings is as follows:
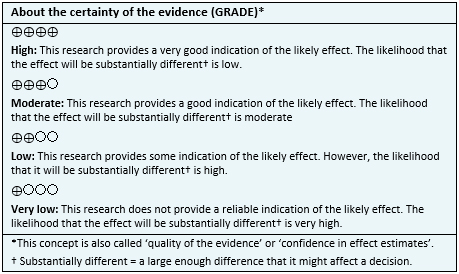
- It is not clear whether cannabinoids have an effect on insomnia severity because the certainty of the evidence is very low.
- Cannabinoids might have no effect on sleep conciliation, but the certainty of the evidence is low.
- It is not clear whether cannabinoids have an effect on sleep quality because the certainty of the evidence is very low.
- Cannabinoids might have no effect on waking sleep and waking behavior, but the certainty of the evidence is low.
- Cannabinoids are probably associated with frequent adverse effects in patients with insomnia. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
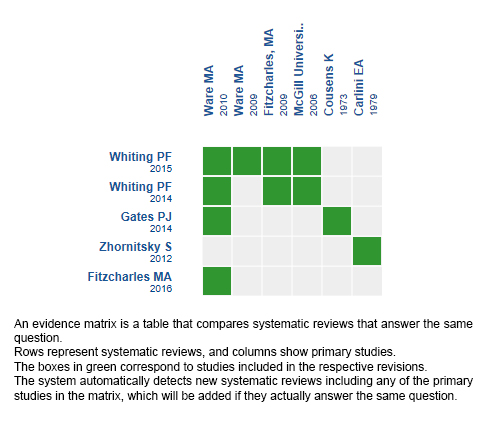
Follow the link to access the interactive version: Cannabinoids for insomnia
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

