Epistemonikos summaries
← vista completaPublished on February 25, 2018 | http://doi.org/10.5867/medwave.2018.01.7155
Is augmentation with folate effective for major depressive disorder?
¿Es efectiva la potenciación con folato para el trastorno depresivo mayor?
Abstract
INTRODUCTION Antidepressant treatment does not lead to a satisfactory response in a significant proportion of patients with depression. It has been postulated that co-administration of pharmacologically standardized nutrients (nutraceuticals), such as folate, would potentiate the effect of antidepressants.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified four systematic reviews including nine studies overall, of which eight were randomized trials. We concluded augmentation with folate for the treatment of major depressive disorder probably results in little or no difference in depressive symptoms. It would be interesting to evaluate the effects of specific presentation forms of folate or in population with objective folate deficit.
Problem
After the introduction of antidepressants in the 1950s, the number of pharmacological treatments for major depressive disorder has increased, but the efficacy of these has remained largely unchanged. Approximately 50% of patients who initiate antidepressant treatment show little or no response after a first trial. Moreover, after several therapeutic approaches, non-remission rates are still around 30% [1],[3]. Adding a drug of a different pharmacological class to the current antidepressant treatment has showed an enhancing effect. Most of the evidence is focused in lithium and atypical antipsychotics. New approaches, such as co-administration of nutraceuticals, in particular folate, as both folic acid and its active presentation (methylfolate), could provide a novel and safer alternative for the treatment of depression [1],[2],[3].
Folate deficiency is a common finding in psychiatric patients and low folate levels have been associated with a worse response to pharmacological treatment. In addition, an association between folate and serotonin metabolism has been observed in patients with congenital defects of the metabolism of the former and in patients with neuropsychiatric disorders. This association could be explained by the role that folate plays in the methylation of homocysteine, necessary for its conversion into s-adenosyl methionine, which has been shown to influence the metabolism of serotonin. Another hypothesis is folate is involved in the methylation reactions of tetrahydrobiopterin, an essential cofactor for the hydroxylation enzymes involved in the synthesis of serotonin [1],[4]. However, the clinical impact of the use of folate as an augmentation strategy in depressive disorders is still controversial.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4], which included nine primary studies overall [5],[6],[7],[8],[9],[10],[11],[12],[13], of which eight were randomized trials [6],[7],[8],[9],[10],[11],[12],[13]. This table and the summary in general are based on the latter, since the observational study did not increase the certainty of the existing evidence or provide relevant additional information. |
|
What types of patients were included* |
Six trials [6],[8],[9],[10],[12] included patients diagnosed with major depressive disorder according to DSM III or IV criteria, and two trials included patients with the same diagnosis according to ICD-10 [7],[13]. Of the eight trials selected, only one [9] exclusively included patients with low folate levels. On the contrary, two trials [6],[7] only included patients without baseline folate or B12 deficiency, and one trial [8] excluded patients with altered laboratory tests (including megaloblastic anemia). The rest of the trials [10],[12],[13] did not report this data. |
|
What types of interventions were included* |
All of the trials used selective serotonin reuptake inhibitors (SSRIs) as antidepressant, except two trials that did not report which antidepressant was used [7],[9]. Methylfolate was used as supplementation in three trials [9],[10] and folic acid in five trials [6],[7],[8],[12],[13]. Six trials compared against placebo [7],[8],[9],[10],[12], one trial compared against antidepressant monotherapy [6] and another trial compared different ranges of folate doses [13]. |
|
What types of outcomes |
The trials measured multiple outcomes, which were grouped by the systematic reviews identified as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information on the effects of folate augmentation for depression is based in four randomized trials [7],[8],[9],[12] that included 591 patients.
Four trials [7],[8],[9],[12] measured the outcome depressive symptoms at the end of the trial (591 patients), one trial [8] reported hospital admission (127 patients) and only one trial [8] reported the rate of adverse effects (127 patients).
The summary of findings is as follows:
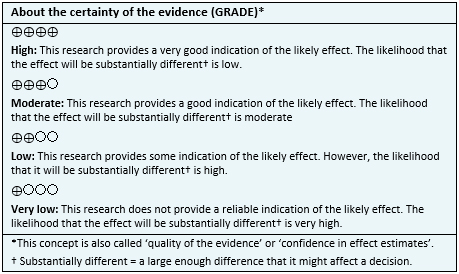
- The addition of folate as augmentation in the treatment of major depressive disorder probably results in little or no difference in depressive symptoms at the end of treatment. The certainty of the evidence is moderate.
- The addition of folate as augmentation in the treatment of major depressive disorder probably results in little or no difference in adverse effects. The certainty of the evidence is moderate.
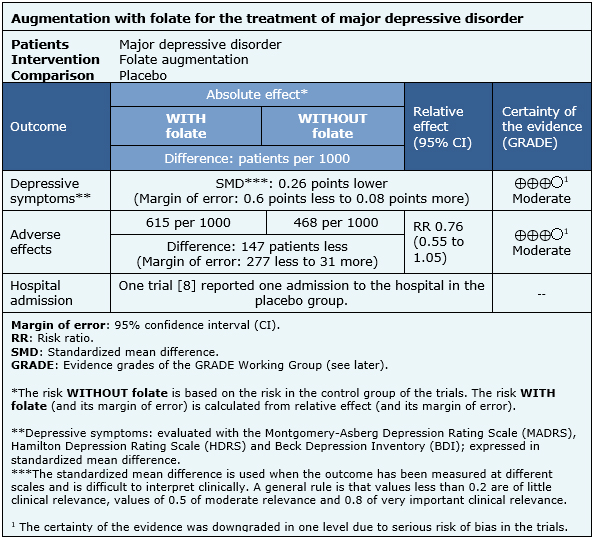
| Following the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
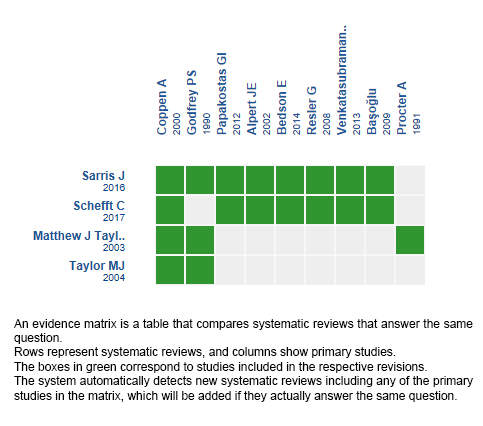
Follow the link to access the interactive version: Folate for depressive disorders
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

