Resúmenes Epistemonikos
← vista completaPublicado el 17 de abril de 2018 | http://doi.org/10.5867/medwave.2018.02.7182
¿Tienen los corticoides inhalatorios un rol en la bronquiolitis?
Do inhaled corticosteroids have a role for bronchiolitis?
Abstract
INTRODUCTION Bronchiolitis consists of an acute small airways inflammation secondary to a viral infection and is a frequent pathology among children under 2 years. The use of inhaled corticosteroids during bronchiolitis has been proposed to reduce recurrent wheeze or asthma, however there is controversy about it.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified three systematic reviews including 11 randomized trials. We concluded that inhaled corticosteroids do not reduce recurrent wheeze or asthma in patients with bronchiolitis.
Problem
Bronchiolitis consists of an acute small airways inflammation commonly caused by a viral infection, predominantly respiratory syncytial virus. It is frequent in children under 2 years and is associated with high hospitalization rates and even mortality. Considering the inflammatory component and the reduction of recurrent wheeze or asthma in other respiratory pathologies with the use of inhaled corticosteroids, they have been proposed to be useful in bronchiolitis.
This article aims to review if inhaled corticosteroids are an alternative to reduce recurrent wheeze or asthma in patients with bronchiolitis.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
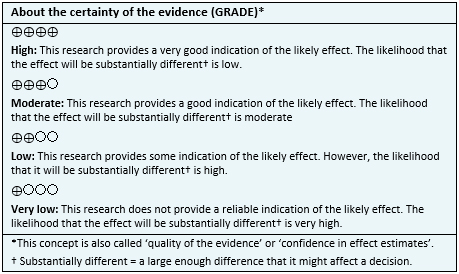
About the body of evidence for this question
|
What is the evidence. |
Three systematic reviews were identified [1],[2],[3], including 11 primary studies [4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14]. All primary studies were randomized trials. |
|
What types of patients were included* |
All trials consider only patients with bronchiolitis. Regarding age, all trials included patients under 24 months. Specifically, one trial included patients aged 9 months or less [8], one trial 42 weeks or less [7] and five trials included patients under 12 months [4],[5],[6],[11],[12],[13]. Mean age was five months, with a range from two to 11 months. Five trials included only patients with confirmed respiratory syncytial virus [4],[6],[8],[13],[14]. In remaining trials, respiratory syncytial virus confirmation varied from 26 to 83% [5],[7],[10],[11],[12]. One trial did not report viral etiology [9]. The main exclusion criteria were: previous wheeze in six trials [6],[10],[11],[12],[13],[14], prematurity in four trials [4],[8],[10],[12], immunodeficiency in five trials [4],[5],[8],[11],[12], previous use of systemic corticosteroids in five trials [4],[5],[6],[12],[14], and assisted ventilation in five trials [5],[7],[8],[11],[12]. All of the trials excluded patients with chronic respiratory or cardiac illness. Seven trials included inpatients [4],[6],[8],[9],[10],[11],[14]. Four trials included outpatients [5],[7],[12],[13]. |
|
What types of interventions were included* |
Seven trials compared inhaled corticosteroids versus placebo [4],[6],[7],[10],[11],[12],[14]. Remaining four trials compared them versus no treatment [5],[8],[9],[13]. Particularly, seven trials used budesonide [4],[7],[8],[9],[10],[11],[13], two used beclomethasone [5],[6], one used fluticasone [12] and one used dexamethasone [14]. In six trials corticosteroids were administered via metered dose inhaler [5],[6],[7],[8],[12],[13]. In remaining five trials, they were nebulized [4],[9],[10],[11],[14]. Regarding intervention length, in two trials it lasted less than 21 days [4],[8], in two it lasted up to 8 weeks [7],[11], in four it lasted three months [5],[6],[12],[13] and in two trials it lasted 4 months [9],[10]. One trial did not report exact intervention length [14]. |
|
What types of outcomes |
Systematic reviews reported the following outcomes: recurrent wheeze, asthma, hospital readmission, need of bronchodilator, length of oxygen supplementation, oxygen supplementation at night, symptoms frequency and adverse effects. Follow-up was three months in one trial [14], six months in one trial [11], a year in six trials [4],[5],[6],[7],[9],[12], two years in two trials [8],[13] and three years in one trial [10]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
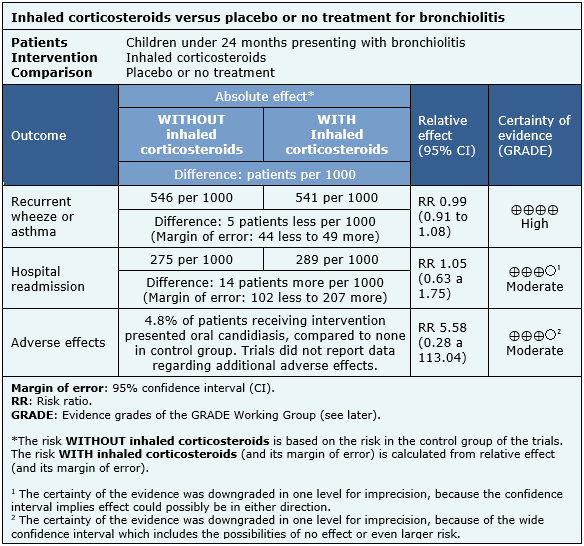
Information regarding the effects of inhaled corticosteroids is based on ten randomized trials including 927 patients [4],[5],[6],[7],[8],[10],[11],[12],[13],[14]. One included trial had no extractable data from systematic reviews, therefore it was not added to a meta-analysis [9]. Seven trials reported recurrent wheeze [4],[5],[6],[7],[11],[12],[14], and three reported asthma [8],[10],[13]. Five trials reported hospital readmission [4],[7],[11],[12],[14]. Three trials mentioned adverse effects [7],[11],[12], but only oral candidiasis could be analyzed from one trial [12].
The summary of findings is the following:
- The use of inhaled corticosteroids does not reduce recurrent wheeze or asthma in patients with bronchiolitis. The certainty of the evidence is high.
- The use of inhaled corticosteroids probably does not reduce the rate of hospital readmissions. The certainty of the evidence is moderate.
- The use of inhaled corticosteroids probably increases importantly the risk of oral candidiasis.
- No additional studies were found reporting information about other adverse effects.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Summary of Findings.
Information regarding the effects of inhaled corticosteroids is based on ten randomized trials including 927 patients [4],[5],[6],[7],[8],[10],[11],[12],[13],[14]. One included trial had no extractable data from systematic reviews, therefore it was not added to a meta-analysis [9]. Seven trials reported recurrent wheeze [4],[5],[6],[7],[11],[12],[14], and three reported asthma [8],[10],[13]. Five trials reported hospital readmission [4],[7],[11],[12],[14]. Three trials mentioned adverse effects [7],[11],[12], but only oral candidiasis could be analyzed from one trial [12]. The summary of results is the following:
· The use of inhaled corticosteroids does not reduce recurrent wheeze or asthma in patients with bronchiolitis. The certainty of the evidence is high.
· The use of inhaled corticosteroids probably does not reduce the rate of hospital readmissions. The certainty of the evidence is moderate.
· The use of inhaled corticosteroids probably increases importantly the risk of oral candidiasis.
· No additional studies were found reporting information about other adverse effects.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
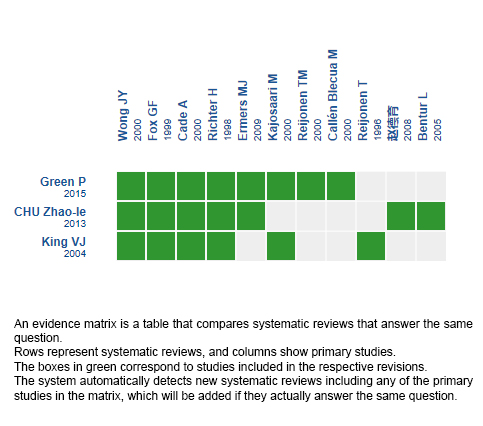
Follow the link to access the interactive version: Inhaled corticosteroids for bronchiolitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

