Epistemonikos summaries
← vista completaPublished on April 4, 2018 | http://doi.org/10.5867/medwave.2018.02.7185
Are probiotics effective in preventing urinary tract infection?
¿Son efectivos los probióticos en prevenir infecciones del tracto urinario?
Abstract
INTRODUCTION Urinary tract infection is the most common bacterial infection and recurrences are common. Probiotics have been proposed as an alternative to decrease this risk. However, it is not clear if they are really effective.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified six systematic reviews including nine studies overall, of which seven were randomized trials. We concluded it is not clear whether probiotics decrease the risk of symptomatic urinary tract infection, because the certainty of the evidence is very low.
Problem
Urinary tract infection is the most frequent bacterial infection. It is associated to important morbidity, such as pyelonephritis, sepsis, abscess and renal failure. It is estimated that 40% of the adult population has presented at least 1 episode of urinary infection, of which 80 % are women. Approximately 20-30% of women with a first episode will experience a recurrence.
Probiotics have been proposed among the alternatives for prophylaxis. They would decrease the risk of urinary tract infection by creating a barrier against infectious pathogens, thus reducing the adherence, growing and colonization of such agents. However, the real efficacy of this intervention is not yet clear.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] that included nine primary studies [7],[8],[9],[10],[11],[12],[13],[14],[15], seven of which correspond to randomized controlled trials [7],[8],[9],[10],[11],[12],[13]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
All of the trials included women only. Two trials included patients with recurrent urinary tract infection [9],[11], four with an acute episode or with an episode of urinary tract infection within last year [7],[10],[12],[13] and one included healthy participants [8]. One trial included patients under 18 years [13] and the rest only included adults [7],[8],[9],[10],[11],[12]. Five trials excluded patients with use of prophylactic antibiotics [7],[9],[11],[12],[13], five excluded patients with concomitant disease [7],[9],[10],[11],[13] and three excluded pregnant women [7],[9],[11]. |
|
What types of interventions were included* |
All of the trials used probiotics as intervention. Three trials used oral probiotics; Lactobacillus GG drink 4 x 108 CFU/100 ml 5 days a week for a year [12]; Lactobacillus GG drink 4 x 107 CFU/100 xml 5 times per month for 6 months [13]; Lactobacillus casei var rhamnosus GR-1 y Lactobacillus fermentum RC-14 1 x 109 CFU/100 ml 1 per day for 60 days [8]. The other four trials used probiotics in vaginal suppositories: Lactobacillus casei var rhamnosus GR-1 7.5 x 108 CFU by suppository twice a week for 26 weeks [9]; antibiotic treatment for 3 days with norfloxacin or cotrimoxazol, then lactobacillus casei var rhamnosus GR-1 plus Lactobacillus fermentum B-54 1.6 x 109 CFU by suppository twice a week during 2 weeks and at the end of the next 2 months [7]; Lactin-V 1 x 108 CFU by suppository 1 per day for 5 days [10]; Lactobacillus crispatus CTV-5 5x108 CFU once a day for 5 days [11]. |
|
What types of outcomes |
The outcomes, as they were grouped by the systematic reviews, were the following: symptomatic urinary tract infection episodes, total adverse effects, intervention withdrawal because of adverse effects and serious adverse effects. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
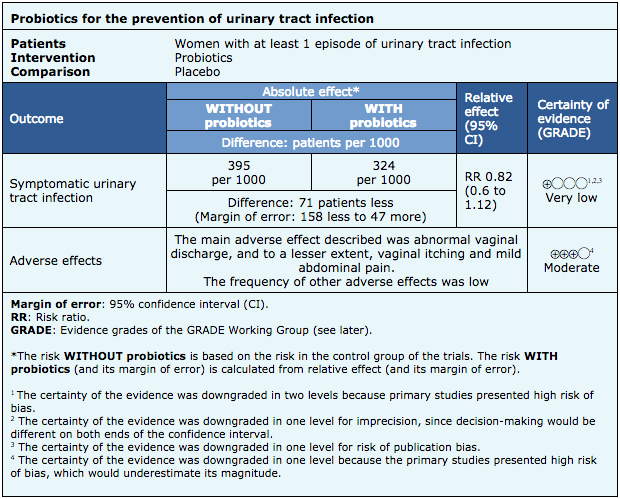
The information about the effects of probiotics is based on six randomized trials [7],[9],[10],[11],[12],[13], that included 352 patients overall. The remaining trial did not report any of the outcomes of interest [8].
All of the trials reported episodes of symptomatic urinary tract infection, three reported total adverse effects [9],[10],[11] and one reported withdrawal due to adverse effects and serious adverse effects [10].
The summary of findings is the following:
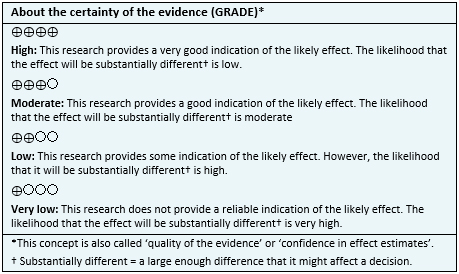
- It is not clear whether probiotics decrease the risk of symptomatic urinary tract infection, because the certainty of the evidence is very low.
- Adverse effects of probiotics (abnormal vaginal discharge) are probably rare. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
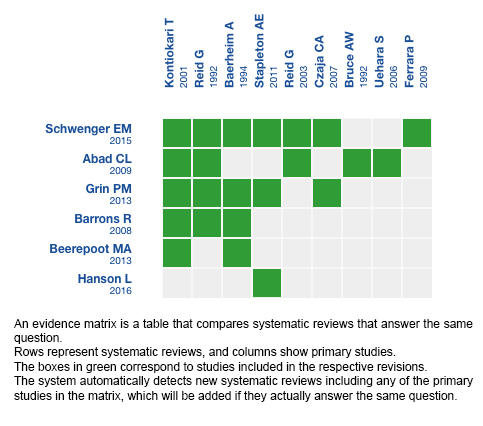
Follow the link to access the interactive version: Probiotics against placebo or no treatment for the prevention of urinary tract infection
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

