Resúmenes Epistemonikos
← vista completaPublicado el 7 de mayo de 2018 | http://doi.org/10.5867/medwave.2018.03.7206
¿Deben utilizarse corticoides sistémicos en la bronquiolitis?
Should systemic corticosteroids be used for bronchiolitis?
Abstract
INTRODUCTION Bronchiolitis is an acute small airways inflammation mainly caused by a viral infection. It is frequent in children under two years of age, particularly under 12 months. The use of systemic corticosteroids has been proposed for bronchiolitis, especially for severely ill patients. However, its efficacy is still controversial.
METHODS To answer this question we gathered information using Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data from primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified four systematic reviews, including 20 randomized trials overall. We concluded the use of systemic corticosteroids has no benefit for the treatment of bronchiolitis, even for patients with mechanical ventilation.
Problem
Bronchiolitis is an acute small airways inflammation mainly caused by a viral infection, being respiratory syncytial virus one of the most important etiologies. It is highly frequent in children under two years of age, particularly under 12 months, being an important cause of hospital admission in this age group. Given its antiinflammatory effects and its efficacy in other respiratory conditions like asthma, the use of systemic corticosteroids has been proposed for patients presenting with bronchiolitis, especially for those severely ill.
The present summary aims to review if systemic corticosteroids are useful as an alternative treatment for patients presenting with an episode of bronchiolitis.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
Four systematic reviews were identified [1],[2],[3],[4]. They included |
|
What types of patients were included* |
All trials included patients presenting with bronchiolitis. Regarding age, 18 trials included patients less than 24 months [5],[6], Four trials included patients with mechanical ventilation [6],[14],[15],[16]. Six trials did not report if they included patients with mechanical ventilation [7],[8],[10],[13],[18],[20]. |
|
What types of interventions were included* |
All trials compared systemic corticosteroids versus placebo. Nine trials used dexamethasone [6],[8],[10],[11],[12],[16],[19],[23],[24], one used prednisone [5], one used methylprednisolone [7], seven used prednisolone [9],[14],[15],[18],[20],[21],[22], one used either prednisolone or methylprednisolone [17], and one used an initial course of hydrocortisone followed by prednisone [13]. In eleven trials the corticosteroid was administered orally [5],[9],[10],[12],[14],[15],[18],[19],[20],[21],[22], in three it was intravenous [6],[8],[16], in four it was intramuscular [7],[11],[23],[24], in one it was either oral or intravenous [17], and in one it was initially intravenous and then oral [13]. In 11 trials, intervention lasted on average four days, with a range from two to ten days [5],[6],[7],[8],[9],[10],[11],[12],[14],[15], |
|
What types of outcomes |
Systematic reviews reported the following outcomes: length of stay in hospital, length of stay in intensive care unit, duration of invasive mechanical ventilation, death of patients with mechanical ventilation, clinical score and duration of symptoms. Follow up was seven days or less in six trials [8],[9],[10],[14],[21],[23], two to four weeks in eight trials [6],[7],[11],[12],[13],[16],[20],[24], two months in one trial [22], a year in one trial [17], two years in one trial [5], and five years in one trial [14]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information regarding the effects of systemic corticosteroids was based on ten randomized trials, including 580 patients [5],[6],[7],[8],[9],[10],[12],[13],[14],[15].Ten trials had no extractable data from the identified systematic reviews [4],[11],[17],[18],[19],[20],[21],[22],[23],[24].Three trials reported clinical score [7],[10],[12], five trials reported length of stay in hospital for patients without mechanical ventilation [6],[7],[9],[12],[13] and two reported length of stay for patients with mechanical ventilation [13],[15]. Three trials assessed the duration of mechanical ventilation [5],[13],[15] and three trials reported mortality in patients with mechanical ventilation [5],[13],[15]. Regarding adverse effects, four trials assessed them [8],[10],[13],[14]. The summary of findings is as follows:
- The use of systemic corticosteroids does not relevantly reduce clinical score for patients with bronchiolitis. The certainty of the evidence is high.
- The use of systemic corticosteroids probably does not reduce length of stay for patients without mechanical ventilation presenting with bronchiolitis. The certainty of the evidence is moderate.
- The use of systemic corticosteroids might result in little or no reduction in length of stay for patients with mechanical ventilation presenting with bronchiolitis. The certainty of the evidence is low.
- The use of systemic corticosteroids might result in little or no reduction of the duration of mechanical ventilation for patients presenting with bronchiolitis. The certainty of the evidence is low.
- The use of systemic corticosteroids might result in little or no reduction of mortality in patients with mechanical ventilation presenting with bronchiolitis. The certainty of the evidence is low.
- Probably, there are no significant adverse effects related to the use of systemic corticosteroids for bronchiolitis.
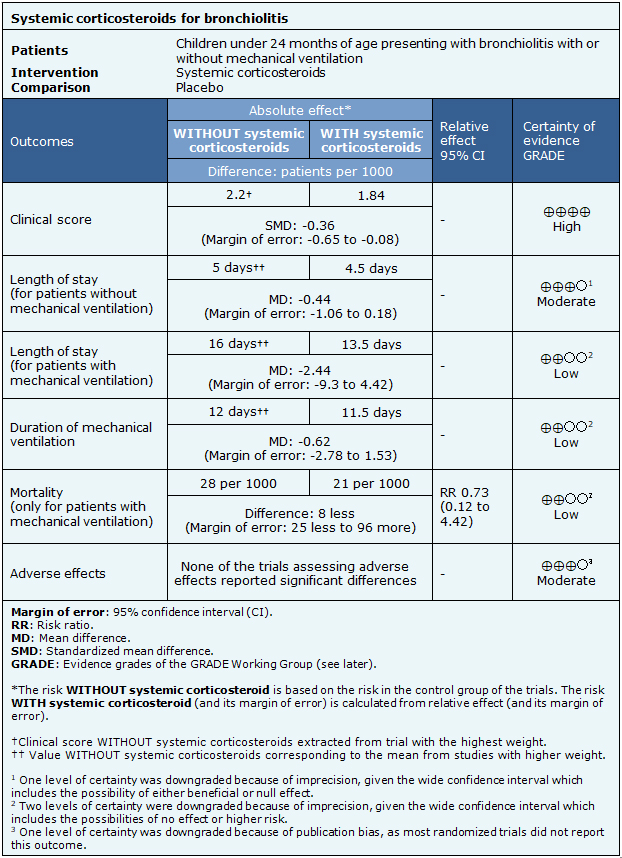
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
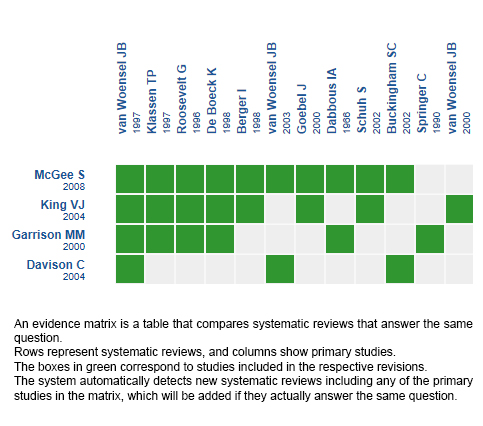
Follow the link to access the interactive version: Systemic corticosteroids for bronchiolitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
● Given the certainty of the evidence, the probability of future research changing the conclusion from this summary is low for patients without mechanical ventilation and moderate for patients with mechanical ventilation.
● During the conduction of this summary, two additional systematic reviews on the use of corticosteroids for bronchiolitis were identified [28],[29]. These were not included, because they did not differentiate inhalatory from systemic routes (oral, intravenous or intramuscular). Related to these reviews, six additional randomized trials were found and not included in the present summary [30],[31],[32],[33],[34],[35]. The systematic reviews analyzed in this summary did not include these trials, because of publication date was beyond the search period, or patients did not meet the inclusion criteria.
● We did not identify ongoing trials in International Clinical Trials Registry of the World Health Organization regarding the use of systemic corticosteroids for bronchiolitis.

