Epistemonikos summaries
← vista completaPublished on September 7, 2018 | http://doi.org/10.5867/medwave.2018.05.7238
Is Ahmed valve superior to Baerveldt implant as an aqueous shunt for the treatment of glaucoma?
¿Es la válvula Ahmed superior al implante Baerveldt como derivación acuosa para el manejo del glaucoma?
Abstract
INTRODUCTION Aqueous shunt has emerged as an alternative technique to trabeculectomy, considered the standard for glaucoma surgery. Currently, it is mainly indicated after failure of trabeculectomy or in glaucoma with high risk of failure. The Ahmed valve and the Baerveldt implant are the most commonly used aqueous shunts. However, it is not clear whether there are differences between them.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified five systematic reviews including 10 studies overall, of which two were randomized trials. We concluded the Ahmed valve probably achieves a lower decrease in intraocular pressure, might lead to less qualified success and probably needs more reinterventions than the Baerveldt implant. Regarding safety profile, the Ahmed valve is not clearly superior or inferior to the Baerveldt implant.
Problem
According to the World Health Organization, glaucoma is the third cause of blindness worldwide [1]. Among the known risk factors for the development of this disease, the intraocular pressure is the only one that can be modified.
Since late 20th century, the introduction of aqueous shunts has emerged as an alternative surgery to trabeculectomy for patients with glaucoma and surgery indication. These devices are made up of a silicone tube with a lumen attached to an explant plate. The main indication of this technique is in those who, having an indication for surgical resolution, have failed to trabeculectomy or have subtypes of glaucoma in which this surgery has a high risk of failure. Currently, the most used techniques are the Ahmed valve and the Baerveldt implant. They differ in that the Ahmed valve has a restricting flow mechanism for preventing postoperative hypotony. However, it is not clear which one is the best alternative.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [2],[3],[4],[5],[6] including 10 primary studies reported in 16 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [21],[22] of which two corresponded to randomized trials, reported in eight references [7],[8],[10],[11],[12],[14],[15],[16]. |
|
What types of patients were included* |
Regarding type of glaucoma, one trial included patients with primary open-angle glaucoma, primary chronic closed-angle glaucoma, neovascular glaucoma and uveitic glaucoma [10] and one trial included patients with primary open-angle glaucoma, primary chronic closed-angle glaucoma, neovascular glaucoma, uveitic glaucoma, post-traumatic glaucoma, combined mechanism glaucoma, congenital glaucoma and penetrating keratoplasty associated glaucoma [14]. |
|
What types of interventions were included* |
Both trials compared the Ahmed FP7 valve versus the Baerveldt 350 mm2 implant and placed them in the superotemporal quadrant [10],[14]. |
|
What types of outcomes |
The trials measured multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up of the trials was 33 months, with a range between 12 and 60 months. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
The information on the effects of the use of Ahmed valve compared to the use of Baerveldt implant is based on two randomized trials involving 514 eyes [10],[14].
Both trials reported mean intraocular pressure at the end of the follow-up (365 eyes) [10],[14], qualified success rate (443 eyes), change of visual acuity (364 eyes), need of reintervention (514 eyes), corneal edema (514 eyes), risk of narrow anterior chamber (514 eyes) and risk of choroidal effusion (514 eyes). One trial evaluated hypotonic maculopathy (276 eyes) [10].
The summary of findings is the following:
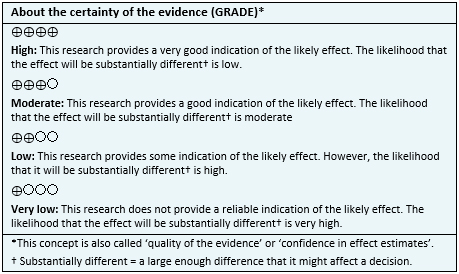
- The use of Ahmed valve probably achieves a lower decrease in intraocular pressure compared to the use of Baerveldt implant. The certainty of the evidence is moderate.
- The use of Ahmed valve might achieve a lower qualified success than the use of the Baerveldt implant, but the certainty of the evidence is low.
- The use of Ahmed valve might be equivalent to the Baerveldt implant in terms of postoperative visual acuity change, but the certainty of the evidence is low.
- The use of Ahmed valve probably increases the need of reoperation compared to the use of the Baerveldt implant. The certainty of the evidence is moderate.
- The use of Ahmed valve probably decreases the development of corneal edema. The certainty of the evidence is moderate.
- The use of Ahmed valve might decrease the development of narrow anterior chamber, but the certainty of the evidence is low.
- The use of Ahmed valve might increase the development of the choroidal effusion, but the certainty of the evidence is low.
- It is not clear whether the use of Ahmed valve increases the development of hypotonic maculopathy, because de certainty of the evidence is very low.
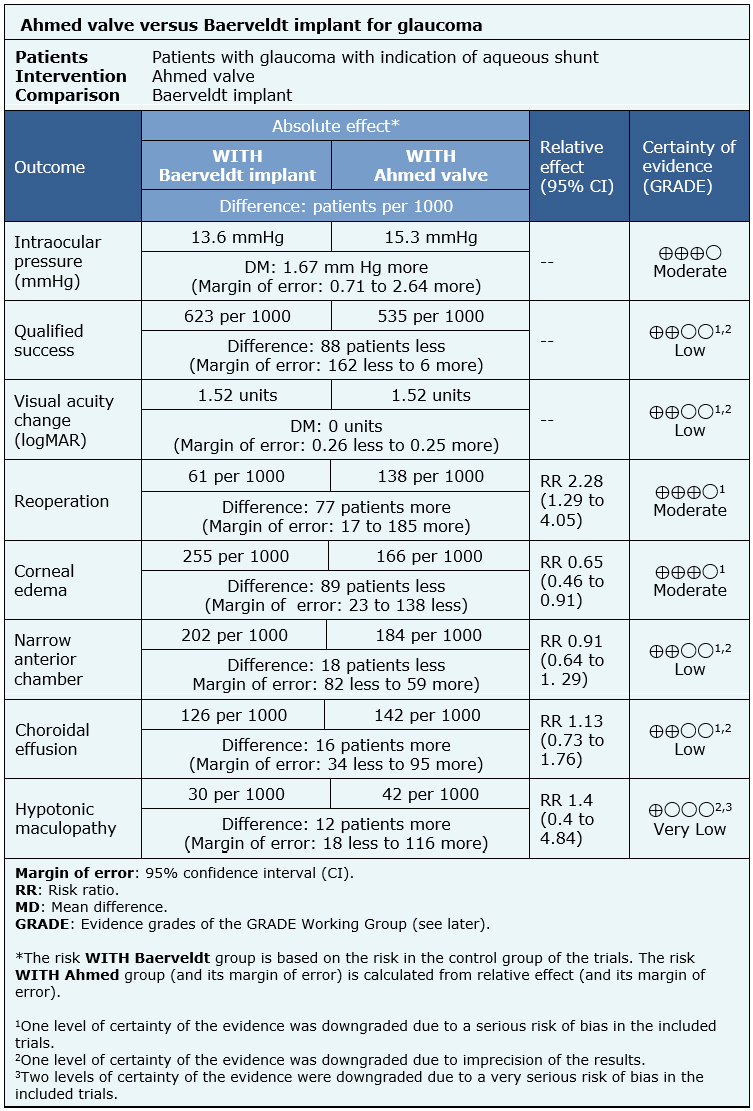
| Following the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
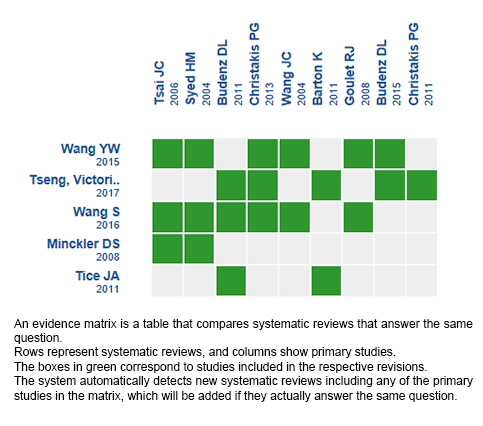
Follow the link to access the interactive version: Ahmed valve versus Baerveldt implant for glaucoma
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

