Epistemonikos summaries
← vista completaPublished on November 12, 2018 | http://doi.org/10.5867/medwave.2018.07.7331
Ranolazine as an additional antianginal therapy in patients with stable symptomatic coronary artery disease
Ranolazina como terapia antianginosa adicional en pacientes con enfermedad coronaria estable sintomática
Abstract
INTRODUCTION There are several effective therapeutic alternatives for stable coronary artery, in terms of prevention of cardiovascular morbidity and mortality. However, the best way to achieve symptomatic control is a matter of debate, particularly in those who do not respond to first-line therapy. This summary aims to evaluate the role of ranolazine as an additional therapy to standard antianginal treatment in patients with persistent symptoms.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified four systematic reviews including 16 studies overall, all of which were randomized trials. We concluded additional treatment with ranolazine might decrease the frequency of anginal episodes but increase adverse effects. It probably has no effect on the risk of death or acute myocardial infarction.
Problem
Coronary artery disease includes a wide range of clinical manifestations, from acute presentations, such as myocardial infarction, to chronic conditions, such as stable coronary artery disease. The latter has a significant impact on both mortality and quality of life. There are several pharmacological alternatives with proven effect on reducing mortality - both cardiovascular and total - and improving the quality of life through symptomatic relief. However, there is a subgroup of patients that does not respond adequately to such therapy.
Ranolazine is a drug that inhibits late sodium currents that are abnormally active in the ischemic myocardiocyte. Its effect prevents intracellular calcium overload (as it is exchanged for sodium) with the consequent diastolic dysfunction that underlies the angina. This is why it has been proposed as an effective therapeutic option in stable coronary disease with suboptimal response to conventional treatment.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a section of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] that included 16 primary studies reported in 19 references [5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16], However, five trials were conducted in patients without stable coronary disease (microvascular angina or acute coronary syndrome) [7],[9],[10],[11],[22]; five used ranolazine as monotherapy [12],[13],[14],[17],[23]; and two measured clinically irrelevant outcomes (time to ST segment depression and mild adverse effects) [6],[16]. This table and the summary in general are based on four randomized trials that used ranolazine as an additional therapy in patients with stable coronary artery disease and reported outcomes critical for decision-making [5],[8],[18],[20]. |
|
What types of patients were included* |
All trials included symptomatic adults with coronary artery disease confirmed by angiography. One trial [20] included patients with ejection fraction less than 40%, while the remaining three excluded patients with severe heart failure (defined as NYHA functional class III or IV) [5],[18],[21]. All trials excluded patients with decompensated cardiac comorbidities (hypertension, pericarditis, among others) or systemic diseases (such as liver damage, chronic renal failure, diabetes). Likewise, three of the trials [5],[18],[21] excluded patients with a history of arrhythmia or comedication with proarrhythmic drugs. The average age of the participants ranged from 61 to 72 years in the different trials. |
|
What types of interventions were included* |
All trials used ranolazine 1 g every 12 hours. Additionally, one trial [20] used standard medical therapy with ranolazine 500 mg every 12 hours; while another trial [21] also used standard medical therapy associated with ranolazine 750 mg every 12 hours. All trials compared against placebo associated with standard treatment. |
|
What types of outcomes |
The outcomes were grouped by the systematic reviews as follows:
The average follow-up of the trials was 5.75 months with a range from 9 weeks to 14 months. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
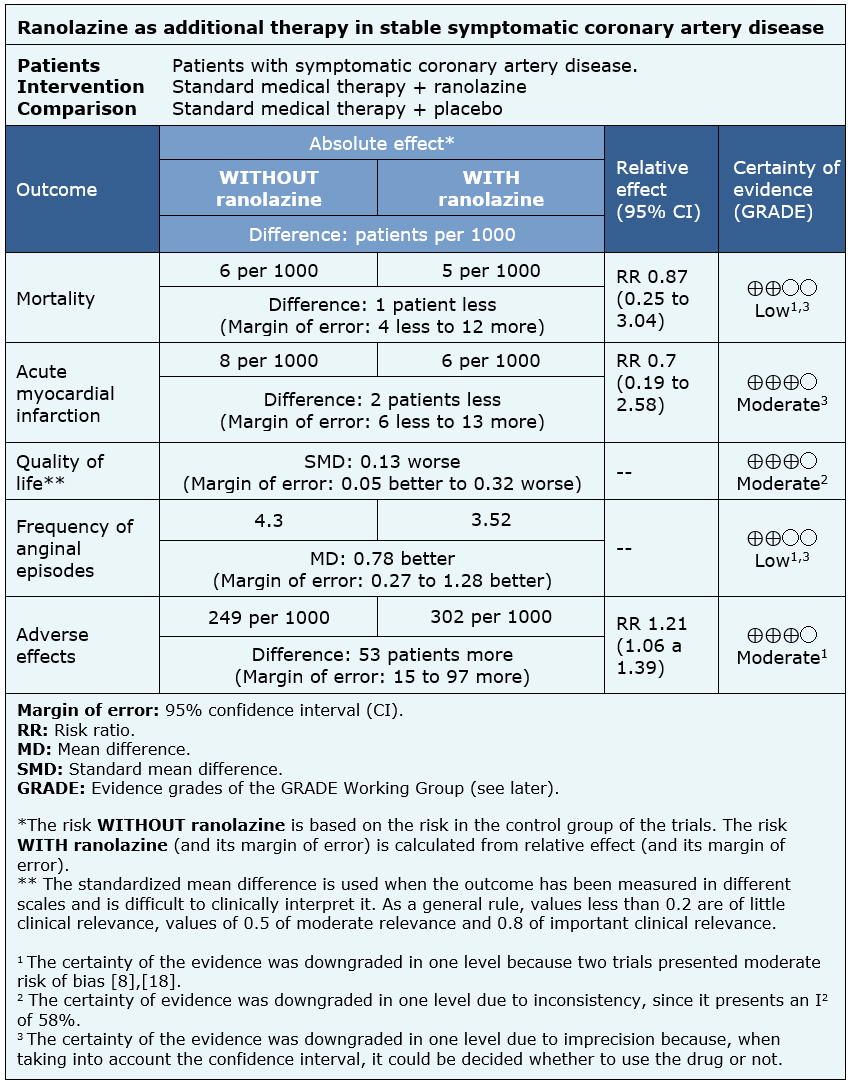
The information on the effects of ranolazine on symptomatic stable coronary artery disease is based on four randomized trials involving 2364 patients.
Three trials measured all cause mortality (2053 patients) [5],[8],[18], three measured quality of life (1533 patients) [8],[18],[20], two reported acute myocardial infarction (1509 patients) [5],[18] and three reported the frequency of anginal episodes (2004 patients) [5],[8],[18].
The summary of findings is as follows:
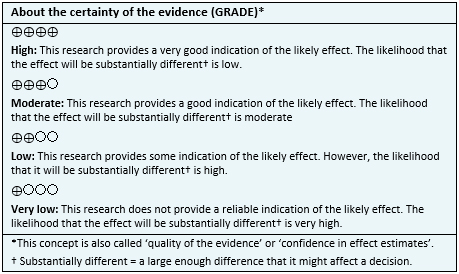
- Ranolazine might lead to little or no difference in mortality in stable symptomatic coronary artery disease, but the certainty of the evidence is low.
- Ranolazine probably results in little or no difference in the incidence of acute myocardial infarction in stable symptomatic coronary artery disease. The certainty of the evidence is moderate.
- Ranolazine probably results in little or no difference in quality of life. The certainty of the evidence is moderate.
- Ranolazine might decrease the frequency of anginal episodes in patients with stable symptomatic coronary artery disease, but the certainty of the evidence is low.
- Ranolazine probably leads to an increase in the incidence of adverse effects. The certainty of the evidence is moderate.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
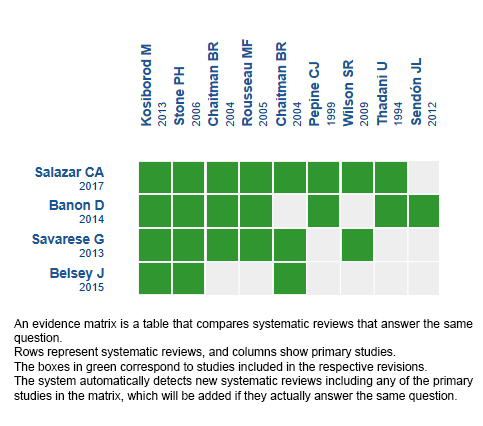
Follow the link to access the interactive version: Ranolazine for ischemic heart disease.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

