Epistemonikos summaries
← vista completaPublished on January 25, 2019 | http://doi.org/10.5867/medwave.2019.01.7511
Preoperative intravitreal bevacizumab for proliferative diabetic retinopathy patients undergoing vitrectomy - First update
Efecto del uso preoperatorio de bevacizumab intravítreo en pacientes con retinopatía diabética proliferativa sometidos a vitrectomía- Primera actualización
Abstract
UPDATE This Living FRISBEE (Living FRIendly Summary of the Body of Evidence using Epistemonikos) is an update of the summary published in December 2014.
INTRODUCTION Proliferative diabetic retinopathy can cause severe vision loss and even blindness if left untreated. Vitrectomy is often required in the treatment of more severe cases. Preoperative administration of bevacizumab, a humanized anti-vascular endothelial growth factor would improve intraoperative variables that facilitate surgery and improve postoperative course.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified five systematic reviews including 16 studies overall, of which 14 were randomized trials. We concluded the preoperative use of intravitreal bevacizumab reduces the rate of vitreous hemorrhage in the early postoperative period, and probably also in the late postoperative period, but its effect on visual acuity is not clear. Furthermore, it probably decreases the surgical time and may decrease the incidence of iatrogenic retinal breaks. Although we are uncertain whether preoperative bevacizumab decreases intraoperative bleeding, it may reduce the need for endodiathermy.
About this update
This Living FRISBEE (Living Friendly Summary of the Body of Evidence using Epistemonikos) is an update of the summary published in December 2014 (doi: 10.5867/medwave. 2015.6160)[1], based on a new systematic review [2] and the update [3] of one of the reviews already included in the previous version [4]. These systematic reviews included four new trials [5], [6], [7], [8] compared to the previous version of this summary.
Considering the new evidence, we have redefined the relevant outcomes, updating and conducting new analyses with the available data. The evidence incorporated in this summary leads to changes related to the certainty of the evidence on the outcomes associated with intraoperative variables, the addition of postoperative outcomes and the reformulation of the adverse effects analysis previously published.
Problem
Proliferative diabetic retinopathy, neovascularization and fibrous proliferation lead to blindness if not treated appropriately. Patients with this condition may have complications such as vitreous hemorrhage, tractional retinal detachment or extensive fibrovascular proliferation requiring vitrectomy as part of their treatment. This procedure has the risk of intraoperative bleeding, which decreases the visibility during the surgery, with the subsequent risk of complications during the postoperative period.
Bevacizumab is a monoclonal antibody directed against vascular endothelial growth factor. Its antiangiogenic properties would be useful in patients with active neovascularization who undergo vitrectomy, facilitating surgery by decreasing intraoperative complications such as bleeding and decreasing the occurrence of vitreous hemorrhage during the postoperative period.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [2], [3], [9], [10], [11] including 16 primary studies [5], [6], [7], [8], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], of which 14 are randomized trials [4], [5], [6], [7], [12], [13], [15], [16], [17], [19], [20], [21], [22], [23]. This table and the summary in general are based on the latter, since the observational studies did not increase the certainty of the existing evidence, nor did they provide relevant additional information. |
|
What types of patients were included* |
Most trials included patients without distinction by sex or age. Only one trial excluded patients under 18 years of age [4]. The age reported by the different trials ranged from 44 [12] to 62 years [5]. All trials included patients with indication of pars plana vitrectomy for complications of proliferative diabetic retinopathy, mainly vitreous hemorrhage [5], [7], [8], [12], [13], [15], [19], [21], [22], [23] and tractional retinal detachment [5], [7], [8], [12], [13], [15], [16], [17], [19], [20], [21], [22], [23]. Only one trial excluded patients with vitreous hemorrhage of any grade [16], while another trial excluded cases of dense vitreous hemorrhage preventing preoperative grading of fibrovascular membranes [5]. One trial did not consider cases of tractional-rhegmatogenous retinal detachment [19] and one trial excluded retinal detachment with macular involvement [20]. There was great variability in the exclusion criteria reported. It should be noted that most trials excluded patients with previous intraocular surgery, especially vitreoretinal surgery [5], [6], [8], [13], [16], [17], [21], [22]; five trials excluded patients with history of myocardial infarction, stroke or thrombotic events [4], [7], [13], [16], [22]; four trials excluded patients with blood coagulopathy, alterations in coagulation tests or use of antithrombotics other than aspirin [13], [16], [21], [22]. Only two trials excluded pregnant women [5], [15] and two excluded previous intravitreal injection of bevacizumab [15], [17]. The trials included 674 eyes in 649 patients, all of them adults. Four trials included more than one eye per patient [5], [8], [19], [21]. |
|
What types of interventions were included* |
All trials compared intravitreal injection of bevacizumab before vitrectomy against vitrectomy alone. Only three trials used simulated injection in the control group [5], [15], [19]. Intravitreal bevacizumab was used in the intervention group in different administration protocols. The dose was mostly 1.25 mg in 0.05 ml [5], [6], [7], [12], [13], [15], [19], [20], [21], [22], [23]. Two trials used higher doses of bevacizumab in the same concentration: one of them used 2.5 mg [17] and another 1.5 mg [16]. The concentration of bevacizumab was the same in all trials except in the most recent one that used a significantly lower concentration with a dose of 0.16 mg in 0.05 ml [8]. The timing of the intervention ranged from 1 to 20 days before surgery. Most trials administered bevacizumab at least 7 days prior to vitrectomy [5], [6], [7], [8], [12], [13], [15], [17], [20], [22]. One trial administered bevacizumab 14 days before the procedure [16]. In two trials patients were randomized to one of three groups: control and two intervention groups, which varied in the preoperative time intervals [19], [21]: 7 or 20 days before vitrectomy respectively [19] and 1 to 14 days prior vitrectomy, with an average of 4.9 days [21]. Data obtained from the 37 eyes of this study [21] receiving intraoperative bevacizumab were excluded when meta-analyses were performed. |
|
What types of outcomes |
There is great variability in the outcomes measured by the trials. These can be classified into intraoperative variables, postoperative course and adverse effects:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
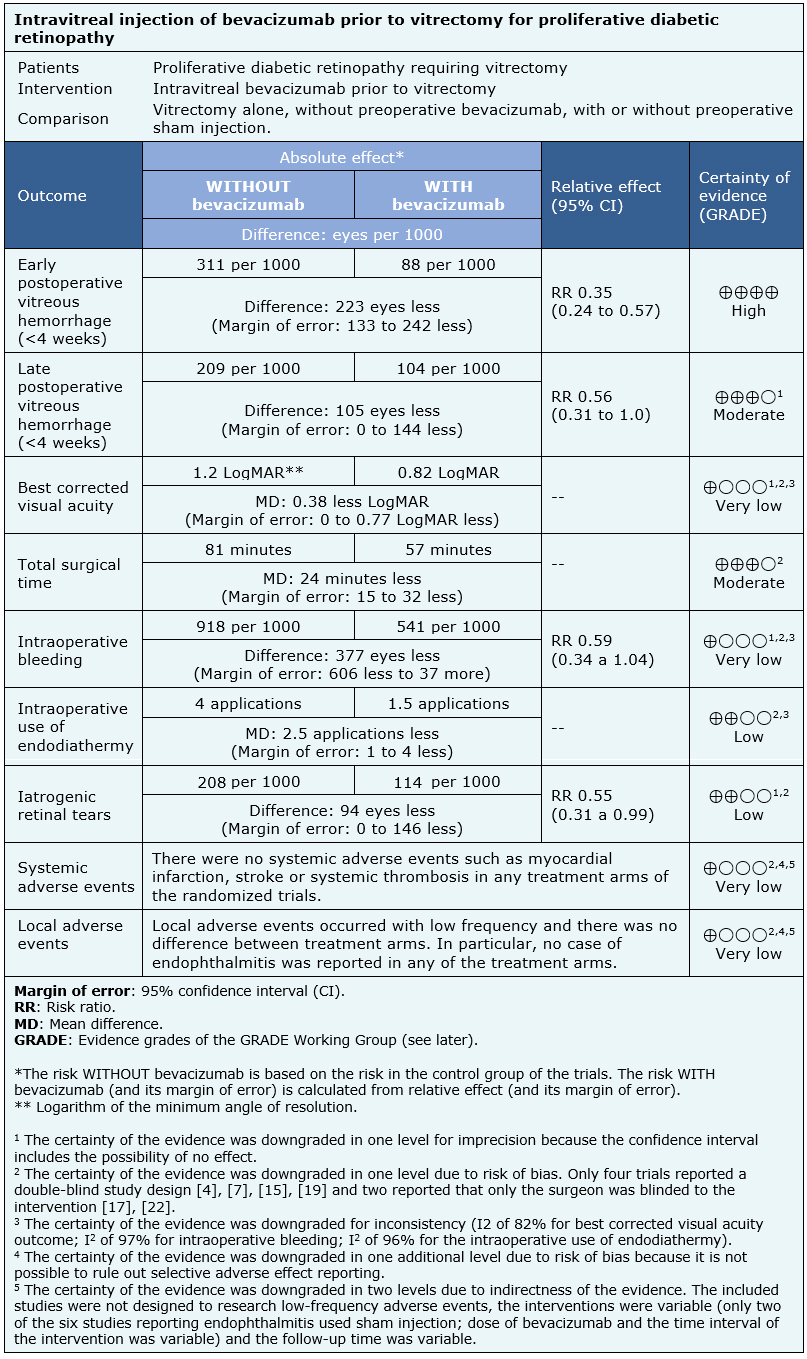
The information about the effects of bevacizumab is based on 13 randomized trials including 674 eyes in 649 patients [5], [6], [7], [8], [12], [13], [15], [16], [17], [19], [20], [21], [22]. One of the trials [23] did not provide information about any outcome of interest.
Nine trials reported early postoperative vitreous hemorrhage (475 eyes) [6], [7], [8], [12], [15], [17], [20], [21], [22] and six reported late postoperative vitreous hemorrhage (269 eyes) [7], [12], [17], [20], [21], [22]. Six trials reported visual acuity during postoperative follow-up (270 eyes) [5], [12], [17], [19], [20], [21], six assessed total surgical time (246 eyes) [8], [12], [13], [17], [19], [20], six intraoperative bleeding (218 eyes) [5], [12], [13], [15], [19], [20], six intraoperative use of endodiathermy (276 eyes) [6], [8], [12], [17], [20], [22] and seven reported iatrogenic retinal tears (333 eyes) [8], [12], [13], [15], [19], [20], [22].
Eight trials assessed systemic adverse events including myocardial infarction, stroke or systemic thrombosis (366 eyes) [5], [8], [12], [13], [15], [16], [19], [21] and six endophthalmitis (318 eyes) [8], [13], [15], [16], [19], [21].
The summary of findings is as follows:
Postoperative course:
- Preoperative use of bevacizumab reduces the incidence of early postoperative vitreous hemorrhage (< 4 weeks) (high certainty evidence).
- Preoperative use of bevacizumab probably reduces the incidence of vitreous hemorrhage in the late postoperative period (> 4 weeks) (moderate certainty evidence).
- We are uncertain whether the use of bevacizumab prior to vitrectomy leads to a change in visual acuity in postoperative follow up equal to or greater than 3 months, as the certainty of the evidence has been assessed as very low.
Intraoperative Variables:
- Preoperative use of intravitreal bevacizumab probably reduces total surgical time (moderate certainty evidence).
- We are uncertain whether the use of preoperative bevacizumab reduces the occurrence of intraoperative bleeding as the certainty of the evidence has been assessed as very low.
- The use of preoperative bevacizumab may reduce the need for use of endodiathermy and the occurrence of iatrogenic retinal tears (low certainty evidence).
Adverse effects:
- We are uncertain whether the use of bevacizumab increases the risk of systemic adverse events such as myocardial infarction, stroke or systemic thrombosis as the certainty of the evidence has been assessed as very low.
- We are uncertain whether the use of intravitreal bevacizumab is associated with a higher rate of local adverse events as the certainty of the evidence has been assessed as very low.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
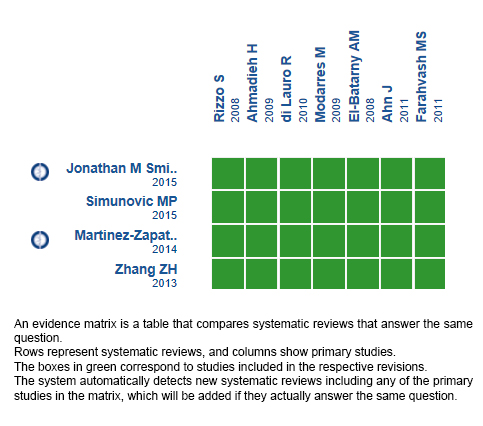
Follow the link to access the interactive version: Preoperative bevacizumab for proliferative diabetic retinopathy.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

