Resúmenes Epistemonikos
← vista completaPublicado el 8 de abril de 2019 | http://doi.org/10.5867/medwave.2019.03.7609
Terapia con presión positiva para enfermedad de Ménière
Positive pressure therapy for Ménière’s disease
Abstract
INTRODUCTION Ménière's disease is a disorder of the inner ear characterized by episodes of spontaneous vertigo, fluctuating hearing loss and tinnitus. Positive pressure therapy has been used to reduce the intensity and frequency of episodes, but it is not clear whether it is actually effective.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified five systematic reviews including 22 studies overall, of which five were randomized trials. We concluded positive pressure therapy probably leads to slightly worse hearing and makes little or no difference in the intensity of vertigo. In addition, we are uncertain whether positive pressure therapy improves functionality or decreases vertigo attacks as the certainty of the evidence has been assessed as very low.
Problem
Ménière's disease is characterized by episodes of spontaneous vertigo, tinnitus and fluctuating hearing loss. The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) guidelines has defined the diagnostic criteria as two episodes of vertigo lasting more than 20 minutes, sensorineural hearing loss confirmed by audiometry and tinnitus or aural fullness [1]. The proposed mechanism of Ménière's disease would be an increase in endolymphatic pressure in the inner ear, whose cause is idiopathic. This would increase the frequency of episodes that, even though may transiently remit, can last several months, with the consequent deterioration in the quality of life [2].
The treatment aims to reduce the number and severity of vertigo episodes, to revert hearing loss and tinnitus, to relieve chronic symptoms and to prevent the progression of the disease. However, there are no treatment alternatives that can achieve these objectives, so positive pressure therapy has been proposed as a minimally invasive intervention.
This therapy uses a device (Meniett) that is inserted into the external auditory canal and produces low intensity pressure pulses. It is believed that these pulses are transmitted to the inner ear, exerting pressure. Before using this device, it is necessary to install a ventilation tube (collar) in the tympanic membrane to allow the passage of waves. This treatment is considered second line and there is still controversy regarding its efficacy in Ménière's disease.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [3], [4], [5], [6], [7] including 22 primary studies [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29] of which five corresponded to randomized trials [14], [18], [20], [23], [27]. This table and the summary in general are based on the latter, since the observational studies did not increase the certainty of the existing evidence nor provide additional relevant information. |
|
What types of patients were included* |
The diagnostic criteria used in four of the trials [14], [18], [20], [27] were those of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) of 1995 for definitive diagnosis of Ménière's disease, specifying certain special characteristics for each of them, while in one trial [23] the criteria were not specified. |
|
What types of interventions were included* |
All trials used the Meniett device or some similar device as intervention, which produced pressure to the middle and inner ear. All trials used a placebo device that was identical to the functioning device, but did not produce pressure. In four trials, the ventilation tube was installed two weeks to two months prior to the beginning of treatment [18], [20], [23], [27]. In one trial it was not reported. [14]. All trials compared against placebo [14], [18], [20], [23], [27]. |
|
What types of outcomes |
The trials evaluated multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up of the trials was 10.5 weeks, with a range between 2 and 16 weeks. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
The information on the effects of positive pressure therapy in Ménière’s disease is based on three randomized trials [18], [20], [27] that included 163 patients in total. The other two trials did not provide sufficient data to be incorporated into a meta-analysis. Two trials [18], [20] reported change in hearing (123 patients),one trial [27] evaluated functionality and frequency of vertigo episodes (40 patients) and one trial reported [20] the intensity of vertigo (68 patients).
The summary of findings is as follows:
- Positive pressure therapy probably leads to slightly worse hearing at four months after starting treatment (moderate certainty evidence).
- We are uncertain whether positive pressure therapy improves functionality as the certainty of the evidence has been assessed as very low.
- We are uncertain whether positive pressure therapy decreases the frequency of vertigo attacks as the certainty of the evidence has been assessed as very low.
- Positive pressure therapy probably makes little or no difference in the intensity of vertigo (moderate certainty evidence).
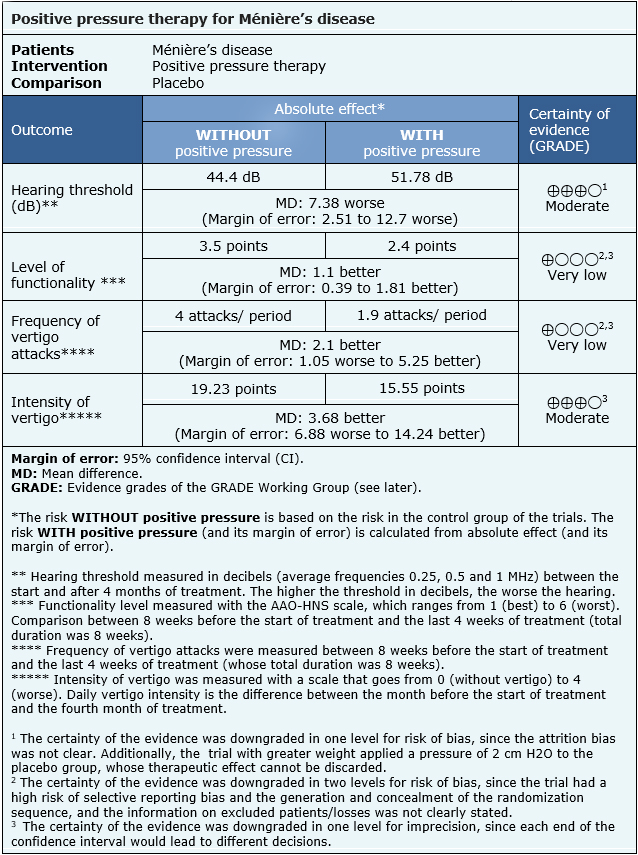
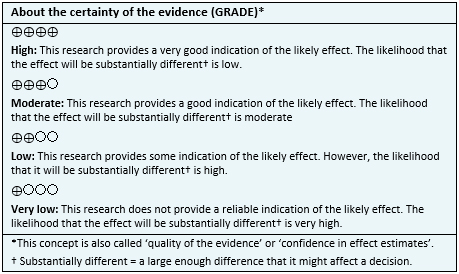
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
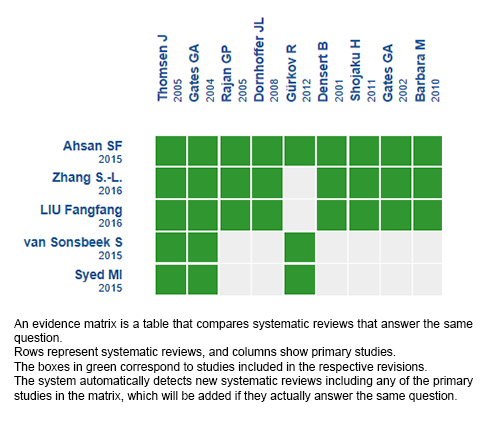
Follow the link to access the interactive version: Positive pressure therapy for Ménière's disease.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

