Epistemonikos summaries
← vista completaPublished on September 11, 2020 | http://doi.org/10.5867/medwave.2020.08.8023
Treat and extend compared to pro re nata regimen in neovascular age-related macular degeneration
Treat and extend comparado con pro re nata en degeneración macular asociada a la edad neovascular
Abstract
INTRODUCTION Age-related macular degeneration is the leading cause of blindness in older people in the world. One of the most effective treat-ments consists of injection intravitreal of anti-endothelial vascular growth factor (anti-VEGF) drugs. However, there is no con-sensus on their frequency of administration, being the treat and extend and the pro re nata the most commonly used regimens, but there is still controversy regarding their effectiveness.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified two systematic reviews that together included two primary studies, both observational studies. We concluded that we are uncertain whether the treat and extend regimen is superior in terms of visual gain, decrease in retinal thickness, number of injections and serious adverse effects at 12 months in comparison with the pro re nata regimen, because the certainty of the existing evidence has been assessed as very low.
Problem
Age-related macular degeneration is the leading cause of blindness in people over 50 years of age [1] and its global projection is estimated to reach 288 million cases in 2040 [2]. The disease is a result of the excessive accumulation of drusen, a residual material that can be found in the macula or peripheral retina, which produces the histological changes leading to macular degeneration [1].
Currently, one of the most effective treatments for neovascular age-related macular degeneration of injection of drugs against endothelial vascular growth factor [3] (anti-VEGF), but there is no consensus in the literature on the frequency of administrations, or under which specific criteria the injections should be applied.
Among its application protocols, we can find the fixed regimes that generally consist of monthly injections, the pro re nata consisting of monthly visits and administration of medication only if there are certain findings on the ophthalmological examination, and thirdly the treat and extend regimen, in which the drug is supplied independent of what is observed by the specialist, but the visit intervals are defined according to the examination, which can range from four to twelve weeks [4].
In this summary, we aimed to evaluate the effectiveness and safety of the treat and extend regimen in comparison with the pro re nata regimen, whose use is still controversial.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified two systematic reviews [5], [6], that included two observational studies [7], [8]. |
|
What types of patients were included* |
Both studies included patients with the diagnosis of neovascular age-related macular degeneration, regardless of stage. One study included patients treated between 2007 and 2008 [7], and one between 2010 and 2014 [8]. One study included only Caucasians with visual acuity over 0.5 (Snellen) [8]. |
|
What types of interventions were included* |
The two studies evaluated treatment with anti-VEGF drugs, comparing treat and extend versus pro re nata regimens [7], [8]. In both studies ranibizumab was used as the anti-VEGF drug in doses of 0.5 milligrams per injection [7], [8]. |
|
What types of outcomes |
The studies reported multiple outcomes, which were grouped by the systematic reviews as follows:
|
* Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
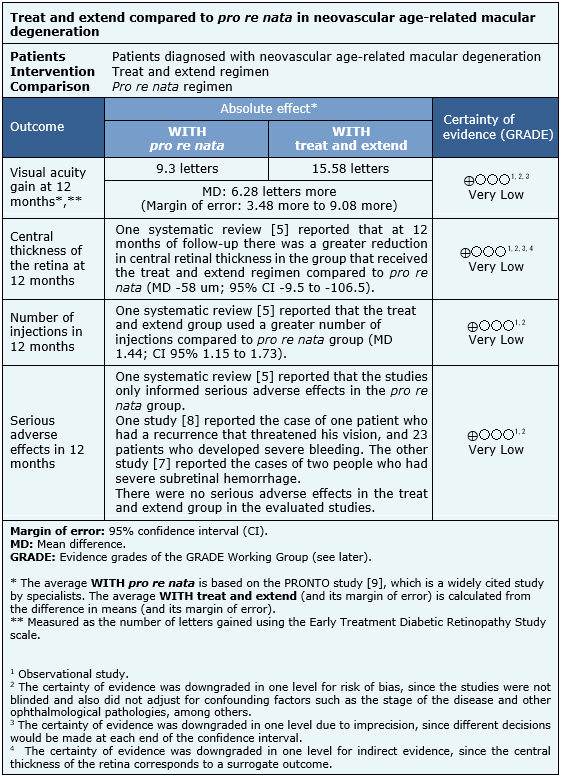
Summary of findings
The information presented on the effects of the use of treat and extend versus pro re nata is based on two observational studies that included 230 patients [5], [6].
The two observational studies measured visual acuity gain at 12 months, serious adverse effects in 12 months and the number of injections in 12 months in each regimen [7], [8] [230 patients]. One study also measured the central thickness of the retina at 12 months (140 patients) [8].
However, no systematic review allowed data extraction of central thickness of the retina, number of injections and serious adverse effects in a way that they could be incorporated into a meta-analysis, so the information of these outcomes is presented as a narrative synthesis of the only review that identified both studies [5].
The summary of findings is as follows:
- We are uncertain whether the treat and extend regimen leads to a greater visual acuity gain at 12 months in comparison with the pro re nata regimen, as the certainty of the existing evidence has been assessed as very low.
- We are uncertain whether the treat and extend regimen leads to a greater decrease of the central thickness of the retina at 12 months in comparison with the pro re nata regimen, as the certainty of the existing evidence has been assessed as very low.
- We are uncertain whether the treat and extend regimen is associated with a greater number of injections in 12 months in comparison with the pro re nata regimen, as the certainty of the existing evidence has been assessed as very low.
- We are uncertain whether the treat and extend regimen is associated with fewer serious adverse effects in 12 months in comparison with the pro re nata regimen, as the certainty of the existing evidence has been assessed as very low.
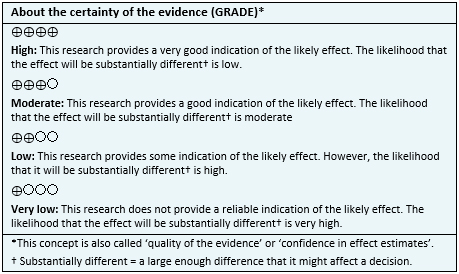
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
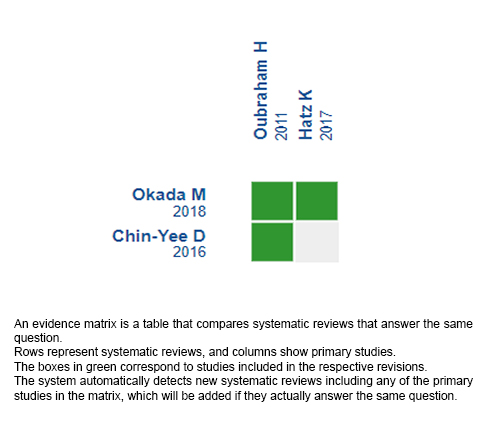
Follow the link to access the interactive version: Treat and extend versus pro re nata in age-related macular degeneration.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

