Resúmenes Epistemonikos
← vista completaPublicado el 9 de abril de 2021 | http://doi.org/10.5867/medwave.2021.03.8046
Quimioterapia paliativa en cáncer de vesícula avanzado
Palliative chemotherapy for advanced gallbladder cancer
Abstract
INTRODUCTION Gallbladder cancer is the most common malignancy of the biliary tract. Given the lack of therapeutic alternatives for advanced stage patients studies have suggested that palliative chemotherapy could benefit these patients.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis, and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified two systematic reviews including two studies overall, of which one was a randomized trial. We concluded that palliative chemotherapy may increase survival in advanced gallbladder cancer patients. However, palliative chemotherapy probably increases adverse effects. In addition, it is essential to carry out a new systematic review, since methodological errors were identified in the analysis and there is new evidence that has not been included in the previous reviews.
Problem
Worldwide, gallbladder cancer is the most frequent biliary tract cancer and the sixth most frequent digestive tract malignancy [1]. An estimated 219,000 new cases and 165,000 deaths were reported worldwide for 2018 [1]. Studies demonstrate that gallbladder cancer incidence and mortality rates display a marked geographical heterogeneity, being especially common in developing countries [2]. The highest gallbladder cancer rates are observed in women from southern Chile (27 per 100,000 habitants) followed by regions of northern India (21.5 per 100,000 habitants). The incidence is relatively uniform in western countries and decreasing (1 per 100,000 habitants) [3]. Gallbladder cancer heterogeneity is mainly attributed to cholelithiasis, the most relevant gallbladder cancer risk factor. As the majority of solid tumors, most gallbladder cancers are adenocarcinomas; on the other hand, more than 50% of patients are diagnosed at advanced stages, with lier, lymph node and/or peritoneal metastases.
Standard of care for localized gallbladder cancer includes surgery. To date, complete surgical resection is the only treatment for gallbladder cancer with curative potential. Adjuvant therapy for patients may include radio or chemotherapy, however their efficacy is still controversial.
On the other hand, in patients with advanced disease, (unresectable, or with distant metastases), local treatments can help to palliate specific symptoms (eg: jaundice), but management is mainly systemic, with chemotherapy. At the moment, no targetable molecular drivers have been identified and, in general terms, the results of treatment are suboptimal compared with other metastatic solid tumors [4]. In this summary, we investigate the effectiveness of palliative chemotherapy in patients with advanced gallbladder cancer, comparing it with the best supportive care.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified two systematic reviews [5], [6] including two studies overall [7], [8], of which one of them was a randomized trial [7]. Finally, this table and the summary, in general, are based on this trial [7] due to the observational study did not increase the certainty of evidence nor did it provide additional relevant information. |
|
What types of patients were included* |
Median age of patients was 49 years, and 80.2% were female [7]. Eligibility criteria [7] were**: Patients with a confirmatory biopsy for unresectable or metastatic gallbladder adenocarcinoma, ≥18 year-olds, with a good bone marrow, renal and liver function, >10 g/dL hemoglobin, normal neutrophil counts, > 100,000/uL platelets, <1.8 mg creatinine, liver enzymes (GOT: glutamic-oxalacetic transaminases and GPT: glutamic-pyruvic) <3 times the normal value, or <5 in case of hepatic compromise diffuse, < 3 mg/dL bilirubin, and 0-2 ECOG (Eastern Cooperative Oncology Group). Patients that had undergone adjuvant chemotherapy or radiotherapy were included if they ended treatments at least 6 months prior to enrollment. |
|
What types of interventions were included* |
Interventions included:
Both schemes were for 6 cycles unless there was disease progression or unacceptable adverse effects (undefined by systematic reviews). Both interventions were compared with best supportive care (BSC), which were not defined by systematic reviews. |
|
What types of outcomes |
The trial reported multiple outcomes, which were grouped by systematic reviews as follows:
|
* Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
**This information was extracted directly from the primary study.
Summary of findings
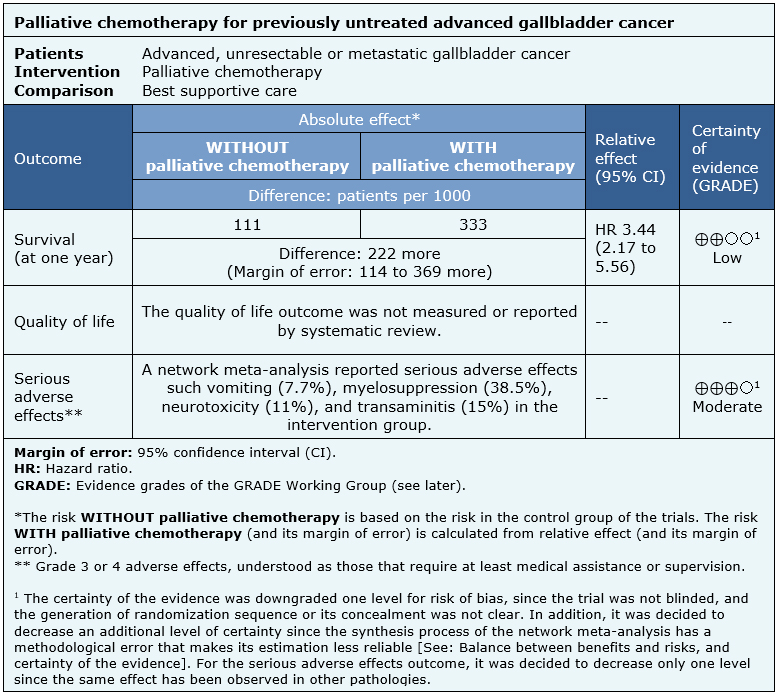
The information about the effects of palliative chemotherapy in advanced gallbladder cancer is based on a randomized trial that included 82 patients [7]. Although the trial had two interventions arms (5-fluorouracil (FU) + folinic acid and gemcitabine + oxaliplatin), the data of the patients treated with 5-fluorouracil and folinic acid (FUFA) were not included as it was considered a chemotherapy regimen scheme based on agents not commonly used for this disease.
Therefore, we decided to reuse the conclusions of a network meta-analysis [5]. One trial measured survival and adverse effects in a group of 53 patients [7]. Quality of life was not reported by systematic reviews.
The summary of findings is as follows:
- Palliative chemotherapy may increase survival in advanced gallbladder cancer (low certainty of the evidence).
- Quality of life was not measured or reported.
- Palliative chemotherapy probably increases adverse effects in advanced gallbladder cancer (moderate certainty of the evidence).
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
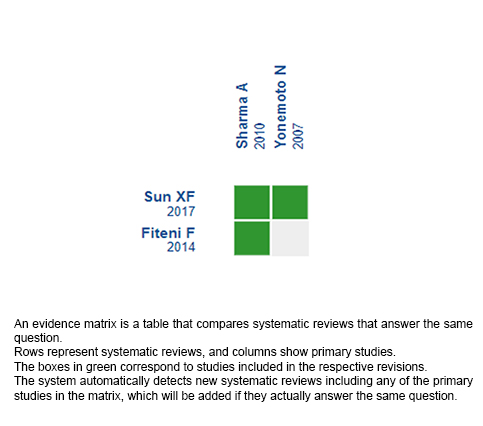
Follow the link to access the interactive version: Chemotherapy versus best supportive care for advanced gallbladder cancer.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

