Resúmenes Epistemonikos
← vista completaPublicado el 7 de mayo de 2021 | http://doi.org/10.5867/medwave.2021.04.8162
Efectos del acetato de ulipristal en pacientes con miomas uterinos sintomáticos
Effects of ulipristal acetate in patients with symptomatic uterine fibroids
Abstract
Introduction Uterine fibroids are frequently encountered in gynecology and are a therapeutic challenge. New therapies, such as ulipristal acetate, could help with symptomatic relief, improve quality of life, and decrease uterine fibroid size. Notwithstanding, there is controversy about adverse effects, especially for hepatotoxicity.
Methods We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis, and generated a summary of findings table using the GRADE approach.
Results and conclusions We identified nine systematic reviews and included ten studies overall, of which five were randomized trials. We conclude that ulipristal increases the likelihood of amenorrhea, improves the quality of life, and decreases menstrual bleeding. However, there is also a likely increase in the risk of adverse effects. Furthermore, ulipristal could decrease the size of fibroids.
Problem
Uterine fibroids have an incidence of 40% in women older than 35 years [1]. They are symptomatic less than 30% of the time, including dysmenorrhea and abnormal uterine bleeding, among the most frequent [2].
Most of the time, these symptoms respond well to medical treatments such as non-steroidal anti-inflammatory drugs or contraceptives. The remaining percentage will eventually require surgery, ranging from myomectomy to hysterectomy. Uterine fibroids account for 60% of major gynecological surgeries [3] and cost between 1,400 and 2,300 USD. When seeking to preserve the patient’s fertility, a myomectomy can be performed, a surgery that generally has high bleeding rates and requires adequate training by the surgeon. Since this technique was described, various preoperative strategies have been tried to reduce bleeding.
Among the different medical alternatives available, ulipristal acetate has been used to reduce bleeding and eventually facilitates surgery [4]. Despite this benefit, there is controversy about its use because adverse events have been described.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), a meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach, and a table of other considerations for decision-making.
|
Main messages
|
In relation to the body of evidence to answer these questions
|
What is the evidence |
We identified nine systematic reviews [1],[4],[5],[6],[7],[8],[9],[10],[11] including 11 studies overall[12],[13],[14],[15],[16],[17],[18],[19],[20],[21],[22] of which five were randomized trials [12],[13],[14],[15],[16],[17]. The table and summary are based on the randomized trials, as the observational studies did neither increase the level of certainty of the evidence nor added any additional relevant information. |
|
What types of patients were included* |
All trials included patients with uterine fibroids, which were defined as the presence of at least one fibroid diagnosed by ultrasound [12],[15],[16], or magnetic resonance [13]. One trial did not define the imaging technique for the diagnosis of uterine fibroids [12]. Of these patients, the age range was 18 to 50 years [12],[15],[16],[17], only one trial considered ages 33 to 50 years [13]. Patients in menopause, using progestins or agents that alter ovarian or liver function, as well as a history of previous gynaecological surgery [12],[13],[15],[16],[17], were excluded. In addition, other trials excluded patients using corticosteroids [12],[13],[17], with a history of gynaecological cancer [12],[13],[15],[17], with coagulation disorders [12],[15],[17], a history of polyp and/or endometrial hyperplasia [12],[17], patients with blood transfusions or haemoglobin less than 6 grams per decilitre [12] and use of anticoagulants [12],[17]. |
|
What types of interventions were included* |
All trials used ulipristal as an intervention and were compared with placebo. Three trials [12],[15],[16] used the drug at doses of 5 and 10 milligrams. Only one trial [13] considered doses of 10 and 20 milligrams. Four trials [13],[15],[16],[17] did not report surgery following ulipristal use. One trial [12] did consider surgery following surgery. |
|
What types of outcomes were measured |
All the clinical trials reported multiple outcomes, which were as follows:
Follow-up ranged from 12 [15],[16] to 13 weeks [12]. Only one trial had a follow-up of 90 and 110 days [13]. |
*Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
What is the evidence
Summary of findings
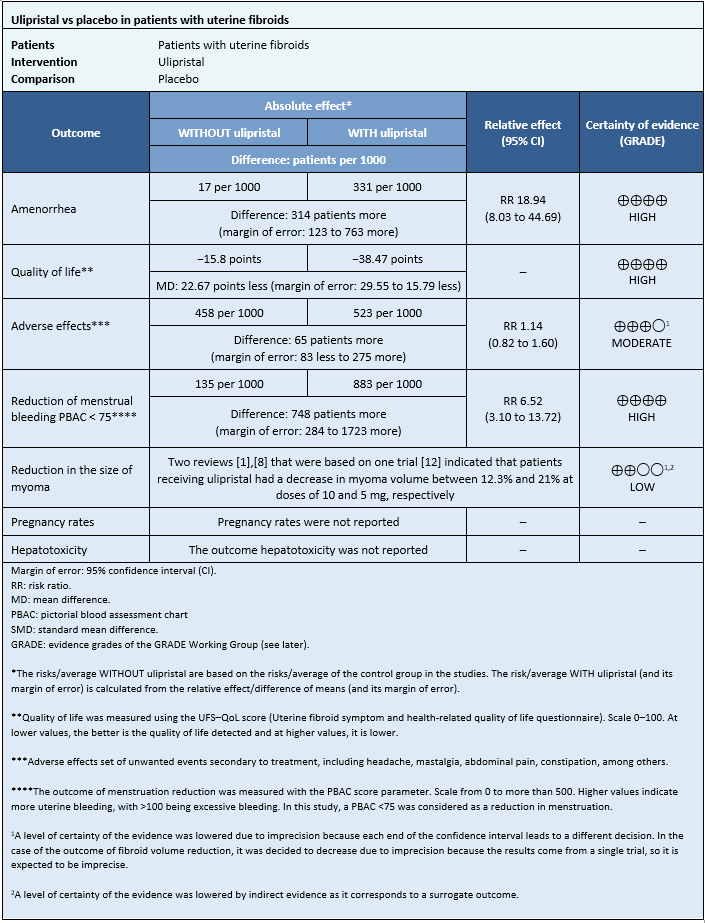
Information on the effects of ulipristal acetate on uterine fibroids is based on four randomized trials [12],[13],[15],[16], which included 873 patients.
Four trials measured amenorrhea outcome (862 patients) [12],[13],[15],[16], two trials measured quality of life outcome (195 patients) [13],[16], and one trial measured adverse effects outcome (237 patients) [12]. Only one trial measured reduction in menstruation at doses of 5 and 10 mg (289 patients) [12]. Only one trial measured fibroid reduction [12].
None of the systematic reviews reported pregnancy rates and hepatotoxicity outcomes.
The summary of results is as follows:
- The use of ulipristal increases the likelihood of amenorrhea in patients with symptomatic uterine fibroids.
- The use of ulipristal slightly improves the quality of life in patients with symptomatic uterine fibroids.
- The use of ulipristal probably increases the risk of side effects.
- The use of ulipristal acetate increases the risk of reduced menstrual flow in patients with symptomatic uterine fibroids.
- It is not possible to clearly establish whether the use of ulipristal acetate decreases the size of fibroids, as the certainty of the existing evidence has been assessed as low.
- No studies were found that evaluated pregnancy rates.
- No studies were found evaluating hepatotoxicity.
Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF)
Other considerations for decision-making
| To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and harms, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
| Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and present it as a matrix of evidence.
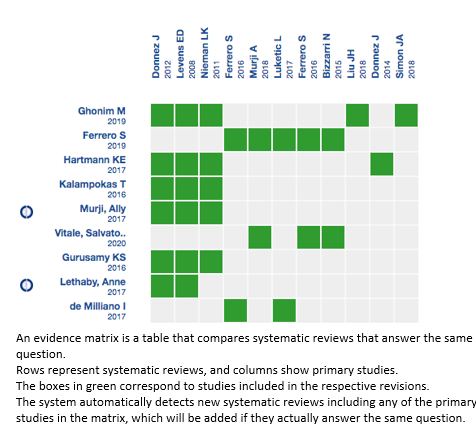
Follow the link to access the interactive version: Ulipristal for uterine fibroids.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time the new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and an internal peer review process. Each of these articles corresponds to a summary, denominated Friendly Summary of Body of Evidence using Epistemonikos (FRISBEE), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a not-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is the Epistemonikos database.
Roles and contributions
RAD: Conceptualization, investigation, writing (review and editing), and supervision. NNP and MVC: Conceptualization, methodology, analysis, investigation, data curation, writing (review and editing), manuscript preparation (original draft preparation), and visualization.
Competing interests
The authors who completed the ICMJE conflict of interest declared that they did not receive funds for completion of this article; they do not have financial relationships with an organization that may have an interest in the published article and they do not have other relationships or activities that may influence the publication of the article.
Funding
The authors declare Universidad Finis Terrae supported this study.
Ethics
This study was not presented to the ethics committee because the source of information for this review was secondary, and the articles were public.
Language of submission
Spanish.

