Resúmenes Epistemonikos
← vista completaPublicado el 28 de abril de 2021 | http://doi.org/10.5867/medwave.2021.03.8171
Vortioxetina para el trastorno de ansiedad generalizada en adultos
Vortioxetine for generalised anxiety disorder in adults
Abstract
INTRODUCTION The currently accepted psychopharmacological treatment for generalised anxiety disorder in adults is associated with several adverse effects which threaten its acceptability. In this line, vortioxetine has been proposed as an alternative with less adverse effects in the treatment of this pathology.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified seven systematic reviews including five primary studies, all corresponding to randomized trials evaluating the effectiveness of vortioxetine in adult patients with generalized anxiety disorder without current treatment. We conclude that there is uncertainty whether vortioxetine increases the response to treatment or improves anxious symptoms, because the certainty of the existing evidence has been assessed as very low. Furthermore, vortioxetine may increase nausea (low certainty evidence).
Problem
Generalised anxiety disorder is a frequent mental illness characterised by persistent anxiety and worry about a wide variety of circumstances, accompanied by a series of physical and psychological symptoms [1]. Globally, generalised anxiety disorder implies a significant burden of disease, detriment of the quality of life and increased health costs [2].
According to the latest clinical practice guidelines, selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors are the first line of psychopharmacological treatment for generalised anxiety disorder in adults [3]. However, due to concerns about its effectiveness and the lack of adherence due to several associated adverse effects [4], new therapeutic strategies have been proposed, such as vortioxetine.
Vortioxetine is a multimodal antidepressant recently approved by the Food and Drug Administration and the European Medicines Agency for the treatment of major depressive disorder [4]. Its mechanism of action—partially elucidated—would be based on the direct modulation of different serotonergic receptors and on serotonin transporter inhibition [4]. Although there is little evidence about its efficacy, vortioxetine has been proposed as a promising alternative in the treatment of generalised anxiety disorder in adults.
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified seven systematic reviews [5], [6], [7], [8], [9], [10], [11] including five primary studies reported in seven references [12], [13], [14], [15], [16], [17], [18], all of which were randomised trials. One of the trials [18] was excluded because it analysed the long-term effectiveness of vortioxetine for generalised anxiety disorder in adults (i.e., relapse prevention), by including patients who had already been treated with vortioxetine in a previous open-label stage, for 20 weeks prior to randomisation. |
|
What types of patients were included* |
All the trials [12], [13], [14], [15] included patients older than 18 years with a diagnosis of generalised anxiety disorder, based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) criteria, without a current treatment. It should be noted that one of the trials [13] delimited the inclusion age up to 65 years. Each randomised trial [12], [13], [14], [15] included patients with greater than or equal score to 20 on the Hamilton Anxiety Rating Scale (HAM-A), and greater than or equal score to 2 in the first two items of the scale (i.e., anxious mood and tension). Similarly, patients with a total score of less than or equal to 16 on the Montgomery – Åsberg Depression Rating Scale were recruited in all the trials [12], [13], [14], [15]. All the trials [12], [13], [14], [15] excluded patients with other psychiatric comorbidities and/or other severe medical illnesses; patients with a recent history of substance abuse or suicidal ideation; and patients who have had a failed response to any previous treatment based on selective serotonin reuptake inhibitors or serotonin and norepinephrine reuptake inhibitors, correctly used (i.e., time and adequate doses) for the current anxiety episode. |
|
What types of interventions were included* |
In all the trials [12], [13], [14], [15] the use of oral vortioxetine once daily was compared with placebo. Three trials [13], [14], [15] evaluated the use of vortioxetine 5 mg per day and two trials [12], [13], [16], vortioxetine 2.5 mg per day and 10 mg per day. |
|
What types of outcomes |
The trials evaluated multiple outcomes, which were grouped by the systematic reviews as follows:
* We considered 'nausea' for the ‘adverse effects’ outcome, due to missing data for total count of adverse effects. |
* Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
Summary of findings
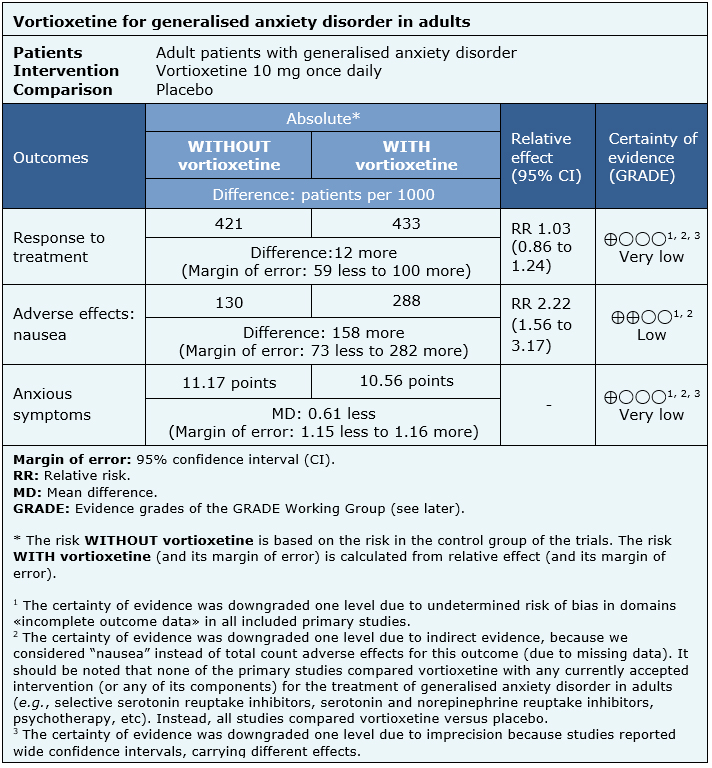
Findings on the effects of vortioxetine for generalized anxiety disorder in adults are based on two randomised trials [12], [13] which included a total of 1238 patients.
This summary are based in two [12], [13] of the four randomised studies included (reported in six references) [12], [13], [14], [15], [16], [17], which evaluated the effectiveness of vortioxetine in adult patients with generalised anxiety disorder, without a current treatment. The above mentioned, because the rest of the trials did not evaluate the use of vortioxetine 10 mg per day, which we considered a matter of interest for the present summary, given their plausibility in clinical practice.
Both trials [12], [13] measured response to treatment (602 patients), adverse effects (nausea) (616 patients), and anxious symptoms (602 patients), comparing vortioxetine 10 mg once daily versus placebo. None of the included systematic reviews allowed data extraction that could be incorporated into a meta-analysis for the outcome remission.
The summary of findings is as follows:
- We are uncertain whether vortioxetine increases response to treatment or if reduces anxious symptoms as the certainty of the evidence has been assessed as very low.
- Vortioxetine may increase adverse effects (nausea) (low certainty evidence).
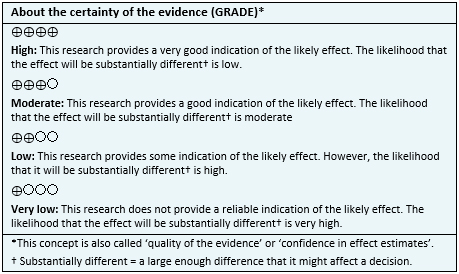
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
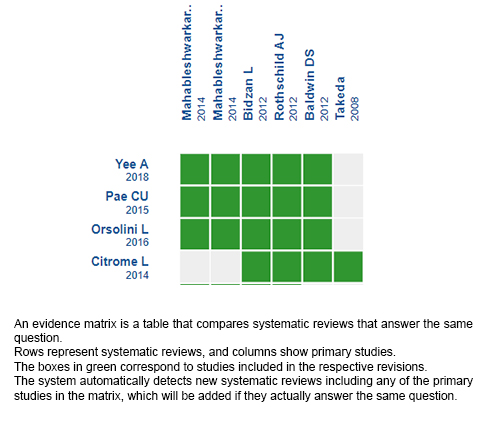
Follow the link to access the interactive version: Vortioxetine for generalised anxiety disorder in adults.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

