Resúmenes Epistemonikos
← vista completaPublicado el 23 de septiembre de 2021 | http://doi.org/10.5867/medwave.2021.08.8460
Adición de terapia fotodinámica a anti factor de crecimiento vascular endotelial en comparación a monoterapia con anti factor de crecimiento vascular endotelial para vasculopatía coroidea polipoidea
Addition of photodynamic therapy to anti-vascular endothelial growth factor drugs compared to anti-vascular endothelial growth factor monotherapy for polypoidal choroidal vasculopathy
Abstract
Introduction Polypoidal choroidal vasculopathy is characterized by multiple and recurrent serosanguineous detachments of the retinal pigment epithelium and aneurysmal protrusions in the choroidal vessels. Different therapeutic options have been proposed, including anti-vascular endothelial growth factor drugs and photodynamic therapy. Controversy exists as to whether combination therapy is superior to anti-vascular endothelial factor drugs alone.
Methods We searched Epistemonikos, the largest database of systematic reviews in health, maintained by screening multiple sources of information, including MEDLINE/PubMed, EMBASE, and Cochrane. We extracted data from the identified reviews, analyzed the data from the primary studies, performed a meta-analysis, and prepared a summary table of the results using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method.
Results We identified three systematic reviews that together included twelve primary studies. Of these, two were randomized trials, and only one of them was included in the analysis.
Conclusions The addition of photodynamic therapy may result in little or no difference in the incidence of retinal hemorrhage and visual acuity gain at six months (low certainty of evidence). On the other hand, the combination of photodynamic therapy and anti-vascular endothelial growth factor drugs compared to anti-vascular endothelial growth factor alone is likely to increase polyp regression at three and six months and reduce central retinal thickness at six months.
Problem
Polypoidal choroidal vasculopathy is considered to be a possible variation of age-related macular degeneration. This pathology is characterized by multiple and recurrent serosanguineous detachments of the retinal pigment epithelium and aneurysmal protrusions in the choroidal vessels.
Although there is no universally accepted definition for polypoidal choroidal vasculopathy, its prevalence is estimated to be 0.3% in the general population and up to 60% among patients with neovascular macular degeneration [1]. This disease typically presents in the late fifth to the sixth decade of life, with unilateral deterioration of visual acuity.
Although a small proportion of patients progress favorably with observation alone, different types of treatments are available. These include focal laser, photodynamic therapy, and anti-vascular endothelial growth factor injections. There is no consensus on whether to use anti-vascular endothelial growth factor monotherapy – which has shown favorable results in final visual acuity – or a combined strategy with photodynamic therapy – which could improve polyp regression. Therefore, a summary of the present evidence is essential to compare both treatments.
Methods
We searched Epistemonikos, the largest database of systematic reviews in health, maintained by searching multiple sources of information, including MEDLINE/PubMed, EMBASE, and Cochrane. We extracted data from the identified reviews and analyzed primary studies. We generated a structured summary called FRISBEE (Friendly Summaries of Body of Evidence using Epistemonikos), following a pre-established format with this information. This format includes: main messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), a meta-analysis of the total studies when possible, a summary table of results using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method, and a section on other decision-making considerations.
|
Main messages
|
In relation to the body of evidence to answer these questions
|
What is the evidence |
We found three systematic reviews [2],[3],[4] that included 12 primary studies [5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16]. Of these, two are randomized trials [8],[14]. One trial [14] includes patients with another diagnosis, so it was not incorporated in the analysis. This table, and the summary in general, is based on a randomized trial [8], as information from observational studies does not increase the certainty of the evidence or add additional relevant information. |
|
What types of patients were included* |
The trial [8] included 40 eyes of 40 patients diagnosed with previously untreated polypoidal choroidal vasculopathy. Of these, 39 completed the study. No exclusion criteria were reported. |
|
What types of interventions were included* |
The trial [8] compared combination therapy with ranibizumab and photodynamic therapy versus ranibizumab therapy as monotherapy. There is also a third group comparing photodynamic therapy in monotherapy in this study, but this was not included in the analysis. The doses used in that work were photodynamic therapy (6 milligrams per square meter) and 0.5 milligrams of intravitreal ranibizumab, versus 0.5 milligrams of intravitreal ranibizumab as monotherapy. |
|
What types of outcomes were measured |
The trials reported multiple outcomes, which were grouped by the systematic reviews as follows:
|
*Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
Summary of findings
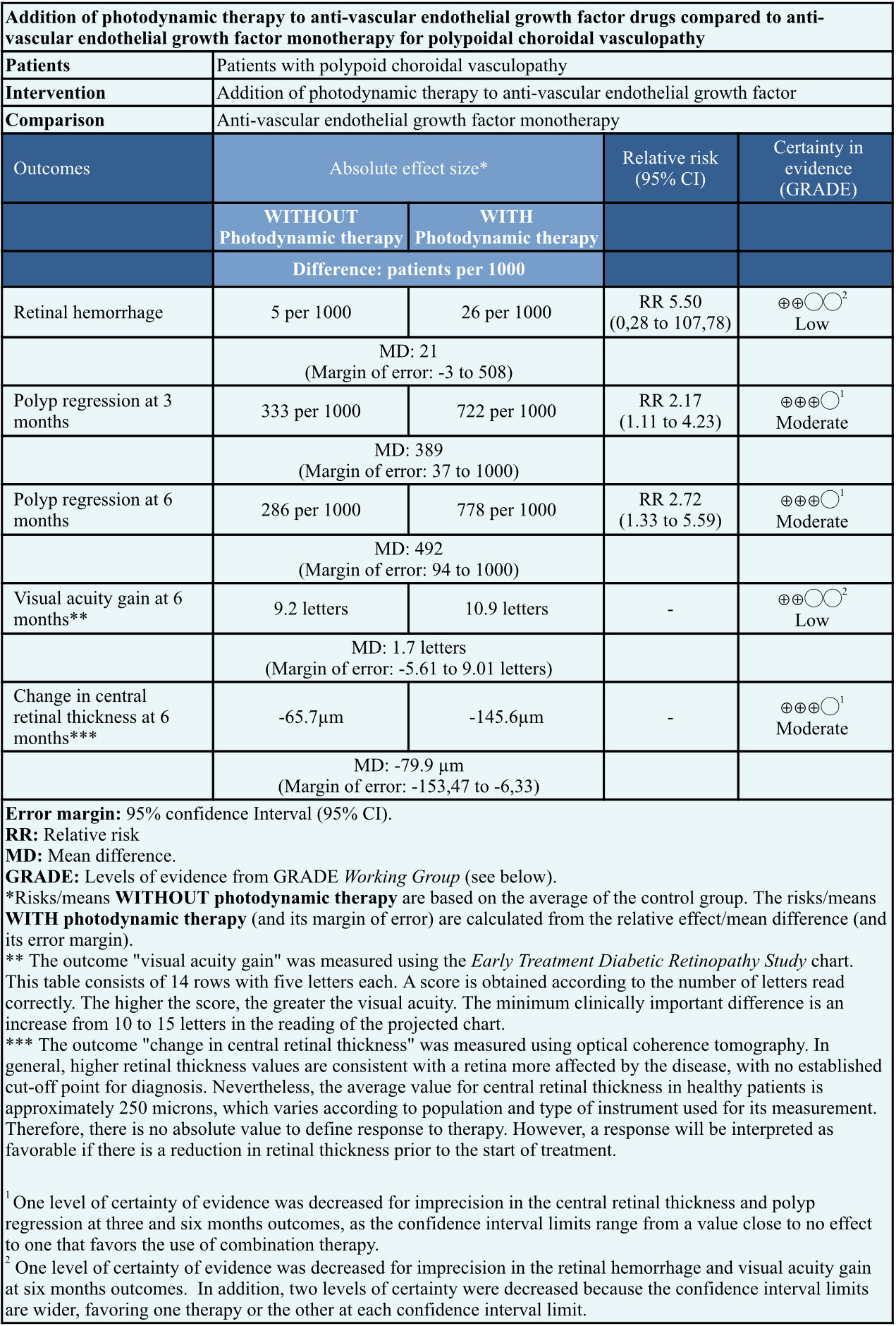
Information on the effects of the addition of photodynamic therapy to anti-vascular endothelial growth factor for polypoidal choroidal vasculopathy is based on one trial involving 39 eyes [8]. This paper measured the outcome of retinal hemorrhage, polyp regression at three and six months, the gain of best-corrected visual acuity at 6 months, and reduction of central retinal thickness at six months. All outcomes in this trial included 39 eyes.
To summarize, the addition of photodynamic therapy to anti-vascular endothelial growth factor drugs compared to anti-vascular endothelial growth factor monotherapy:
- May result in little or no difference in the risk of retinal hemorrhage (low certainty of evidence).
- Is likely to increase polyp regression at three and six months.
- May result in little or no difference in visual acuity gain at six months (low certainty of evidence).
- Is likely to reduce central retinal thickness at six months.
Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF)
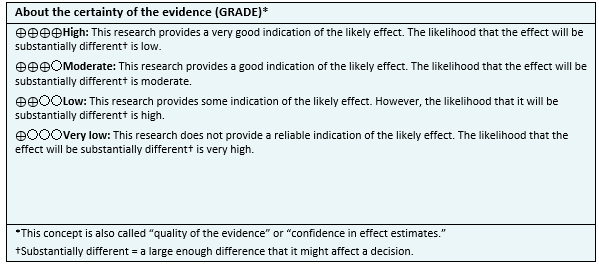
Other considerations for decision-making
| To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Harm/benefit balance and certainty in evidence |
|
| Costs |
|
| What do patients and their caregivers think |
|
| Differences between this summary and other sources |
|
| Could this information change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and present it as a matrix of evidence.
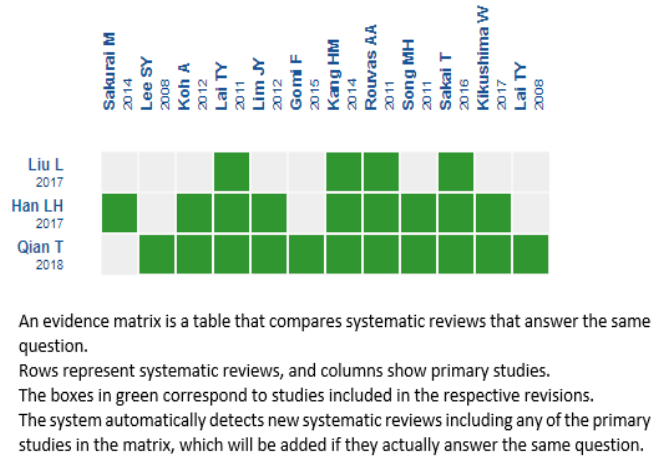
Follow the link to access to the interactive version: Anti-VEGF + photodynamic therapy versus anti-vascular endothelial growth factor monotherapy for polypoidal choroidal vasculopathy.
Notes
If new systematic reviews on this topic are published after this summary's publication, a "new evidence" notification will be displayed at the top of the matrix. While the project provides regular updates of these summaries, users are invited to comment on the Medwave website or contact the authors by e-mail if they believe evidence warrants an earlier update.
After creating an Epistemonikos account, you will receive automatic notifications whenever new evidence potentially answers this question by saving the matrices.
This article is part of the Epistemonikos evidence synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and an internal peer review process. Each of these articles corresponds to a summary, called FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence of a specific question in a friendly manner for physicians. The main resources are based on the Epistemonikos evidence matrix, and the result analysis is based on the GRADE methodology. Further details of this FRISBEE elaboration method are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997).
The Epistemonikos Foundation is an organization that seeks to bring information closer to health decision-makers through the use of technologies. Its main source is the Epistemonikos database (www.epistemonikos.org).
Contributor roles
FMY: methodology, validation, formal analysis, research, resources, data curation, draft writing, review and editing, visualization, fund acquisition. VMV: methodology, validation, formal analysis, research, resources, data curation, draft writing, review and editing, visualization, fund acquisition. RGC: conceptualization, methodology, validation, formal analysis, research, resources, data curation, draft writing, review and editing, visualization, supervision, project management, fund acquisition. AMQ: conceptualization, methodology, validation, resourcing, review and editing, visualization, supervision, project management, fund acquisition.
Competing interests
The authors declare that they have no conflicts of interest.
Funding
The authors declare that they had no external sources of funding for this study.
Ethics
The present study did not require evaluation by an ethics committee, given that it uses secondary data sources to summarize current evidence.
Language of submission
Spanish.

