Análisis
← vista completaPublicado el 6 de marzo de 2025 | http://doi.org/10.5867/medwave.2025.02.3044
Innovación y bioética en cirugía: redefiniendo los límites para un futuro seguro y centrado en el ser humano
Innovation and bioethics in surgery: Redefining boundaries for a safe and human-centered future
Abstract
Since surgery is a complex procedure due to multiple factors, it is more difficult to rigorously evaluate innovative processes in this field than clinical trials of new drugs. Being able to carry out an adequate study design with all its corresponding implications, achieving high-quality standards for these studies, ensuring respect for patients' rights, and verifying that their principles of beneficence, minimization of the risk of harm, justice and autonomy are a challenge for many researchers and professionals involved in the surgical process. Hence, it is advantageous to have guides that guarantee the methodological quality of research on innovative surgical procedures and that these guides include the ethical aspects involved in each of their stages. This review aims to make a historical overview of what has been published on the ethical approach to surgical innovation. In addition, the ethical aspects of the stages of the IDEAL framework for surgical innovation will be explained.
Main messages
- The complexity of surgical innovation poses significant ethical and methodological challenges, as it is harder to rigorously evaluate new procedures compared to clinical trials for medications. It is essential to establish ethical guidelines and frameworks, such as the IDEAL model, to ensure methodological quality and patient safety in surgical advancements.
- Informed consent is a cornerstone in surgical innovation, enabling patients to understand the risks, benefits, and alternatives of new procedures. This is especially critical for vulnerable populations, where alternative approaches must be implemented in emergencies or when cognitive limitations are present.
- Despite initiatives like the IDEAL framework to assess surgical innovations, its global adoption remains limited. There is a pressing need for specific regulatory frameworks and innovation committees to ensure that surgical advancements adhere to ethical principles of beneficence, justice, and autonomy.
Introduction
Surgical specialists constantly adapt their techniques to incorporate new drugs, methods, and ways to control side effects. Unlike other fields, surgery often requires rapid adjustments to unexpected anatomical variations arising from the morphological changes caused by disease. Surgeons must modify their approach on the spot, relying on their experience and skill. Surgery’s dynamic nature reflects the field’s commitment to continual progress, as innovation is integral to surgical development.
Surgery has some advantages due to the surgeon’s ability to resection diseased organs or tissues to remain disease-free. There is no biological resistance to surgical procedures, and they have an excellent cost-benefit ratio. The advent of scientific experimentation on humans through controlled clinical experiments in surgery solidified this paradigm shift in 1899 with the Halstedian paradigm [1]. This has led to a new conception of the modern surgical specialist: to understand more about biology, anatomy, and development of the molecular innards of the diseases we treat. Today, we only accept surgical treatments that demonstrate precision and certainty through evidence-based surgery [2].
From the very origins of the practice of medicine, Hippocrates made it clear that one of the fundamental determinants in the prognosis of any pathology is the doctor who cares for the condition that afflicts the patient. In his epidemic treatise, he mentioned that “the art has three factors: the disease, the patient, and the doctor” [3]. This ruling is definite in the field of surgery, and now, with evidence-based medicine, it can be demonstrated quantitatively in terms of complications and mortality. This is why it is evident that the nutritional and metabolic status of the patient is vital in the prognosis and recovery of surgical patients.
The surgical act is, ultimately, a risk situation, understood as the probability of a negative outcome, as can be a complication, disability, functional alteration of an organ, or even death [4].
Objectively, we can calculate this possibility using the risk-benefit ratio, which recommends that surgery should not be performed if it has more risks than benefits. Members of the community of doctors and sick people, or patients, are increasingly aware of this situation and understand that the proper performance of the surgical procedure is one of the keys to its success. Innate surgical skills and those developed through appropriate training are the essential ingredients to have good results in surgical treatments, with accurate, compassionate judgment, good communication, clinical proficiency, and, above all, teamwork.
In the 20th century, significant advancements occurred in establishing patient rights and shaping their relationship with healthcare professionals and systems. Europe reinforced this shift through the Oviedo Convention, fostering a new approach to the patient-doctor relationship centered on individual autonomy [5]. Thus, decision-making in the context of health has moved from the paternalistic model, which has characterized the history of medicine, to a consensual action between the doctor and the patient. This consolidated advance in the bioethical context has gradually transcended the legal context [6]. Since the 1980s, various laws have incorporated ethical principles into the duties of healthcare professionals and systems, especially regarding patient information [7]. This focus on information is essential to developing surgical techniques, materials, and instruments. It is foundational to the doctor-patient relationship, which respects the patient’s autonomy in making decisions about their body. In this ethical and legal framework, informed consent is the patient’s right to receive clear information about a proposed surgical procedure’s nature, purpose, risks, and benefits. This allows patients to give their informed authorization and approval before undergoing surgery.
Given the complexity of surgical innovation and the accompanying ethical challenges, this review aims to provide a historical analysis of ethical guidelines in surgery, highlighting the evolution of ethical principles in this field. Furthermore, it will explore the specific ethical aspects at each stage of the IDEAL framework, offering a structured approach for ethical evaluation in surgical practice.
Ethical development of surgical innovation
Historically, medical ethics and the commitment to advancing knowledge were shaped by the Code of Hammurabi, the Hippocratic Oath, and the Prayer of Maimonides [8]. In 1543, the Royal College of Physicians in England formalized a code of ethics. In 1628, in New Spain, professional practice regulations were established. These developments, along with contributions from figures like William Harvey (1628), John Hunter (1789), and Joseph Lister (1865), laid the foundations for scientific and experimental surgery [9].
Medicine is an inexact science, and surgical procedures are not fixed; they are performed by humans who remain vulnerable to errors despite experience and ethical commitment. Reducing such errors requires refining surgical methods, with iatrogenic mistakes carefully analyzed through a bioethical lens. Bioethical codes should guide surgical practice, prioritizing patient health, while Ethics Committees in hospitals serve as moral guides, integrating scientific and epidemiological principles into medical conduct [10]. However, laws, procedures, and teaching methods alone cannot ensure positive outcomes without a commitment to bioethics principles. A balanced respect for patient and surgeon rights is essential, encouraging self-critique and focusing on continuous improvement. Surgeons must also be vigilant about infection risks associated with surgical procedures. The high costs of medical technology have posed ethical challenges by making quality care unaffordable for many, which has led to the creation of various healthcare programs in public institutions, private medicine, and now prepaid plans through commercial insurance companies. However, these companies often prioritize profit, selecting institutions and specialists without guaranteeing high-quality medical care [11].
Freedom is the basis of ethical conduct, allowing surgeons to act in ways that can be either moral or immoral. Each surgeon’s values shape their choices, defining them as professionals committed to ethical standards under moral philosophy. Surgeons must consider their actions' legal and ethical implications, especially those connected to life and the advancement of medical and biological sciences. Therefore, ethical principles in surgery and technological innovation should guide professional behavior, forming a code of moral duty mandated by society, law, and educational institutions [7]. This responsibility strengthens the doctor-patient-family relationship, which is crucial for successful outcomes.
In emergencies, certain factors become critical: the primary principle of preserving life, the understanding that most patients want their lives safeguarded, even without prior consent, and the imperative to reduce mortality rates, particularly in complex or extensive procedures [12]. The necessity for ethical guidelines and regulations concerning surgical innovation is paramount, particularly as it pertains to new techniques, modified strategies, or innovative instruments. Unlike the rigorous standards applied to Randomized Controlled Trials (RCTs) for new drug applications, the scientific evidence supporting new surgical methods is often less robust [13]. This discrepancy raises concerns about using uncontrolled studies in surgical innovation, contrasting sharply with the stringent ethical and regulatory frameworks governing pharmaceutical development. The importance of randomized controlled trials in providing scientific validity for new drugs is well established, highlighting the need for similar rigor in evaluating surgical innovations. However, several surgeons consider that innovation studies in surgery do not fit this format [14,15]. This is because it is more difficult to measure the results obtained by surgical techniques than those using new drugs. Furthermore, studies analyzing surgical procedures are more complex to reproduce due to patient characteristics and the disposition of the surgeon, among others. Another fundamental factor that complicates the evaluation of these interventions is the constant innovation in surgery. This is well illustrated by the American Society of University Surgeons, which says that surgeons are trained to perform continuous situational evaluations, decision analysis, and improvisation in preparing for the challenges and creativity required with each clinical case [16].
In the United States, unlike drugs and medications, innovative surgical procedures are not regulated by agencies like the Food and Drug Administration or Institutional Review Boards. Determining the need and timing for a randomized controlled trials to evaluate a new surgical technique is challenging; conducting it too early may restrict innovation while doing it too late risks oversight. Additionally, no regulations prevent surgeons from offering new procedures to patients outside of randomized controlled trials, which complicates standardized evaluation [17]. Assigning patients to case and control groups can be complicated since surgeons and patients strongly prefer an intervention. This would often imply one or both of them refusing to participate in the trial [18]. Another aspect affecting surgeons' participation in surgical trials is their response to uncertainty. Previous negative experiences and threats of legal proceedings could make surgeons reluctant to subject parts of their practice to evaluation. Regarding informed consent, patients must be given sufficient information about the procedure, which is not always feasible [19].
The time of randomization must be very close to the intervention to avoid strong participant preferences, knowledge of the allocation in the randomized controlled trial, and clinical events before the procedure affects this phase of the study. It is essential to mention that regardless of the time at which randomization is done, the surgeon may decide that a surgical procedure is inappropriate, impossible, or unsafe even after randomization. When masking does not exist, this can lead to performance, attrition, and detection biases. Blinding surgeons, patients, and other caregivers are often tricky in surgical trials. However, there are innovative masking methods [20]. Placebo surgery is controversial and has been restricted to cases where no adequate comparator was available or if placebo surgery had limited risk [21]. Another very particular aspect is the complexity of the surgical acts, which require an appropriate evaluation. These procedures involve several components that cannot be separated from each other [22]. Furthermore, the act entails special attention since it depends on several health professionals and encompasses other aspects of healthcare delivery in ways that a pharmacological intervention does not (Figure 1).
Surgical intervention complexity.
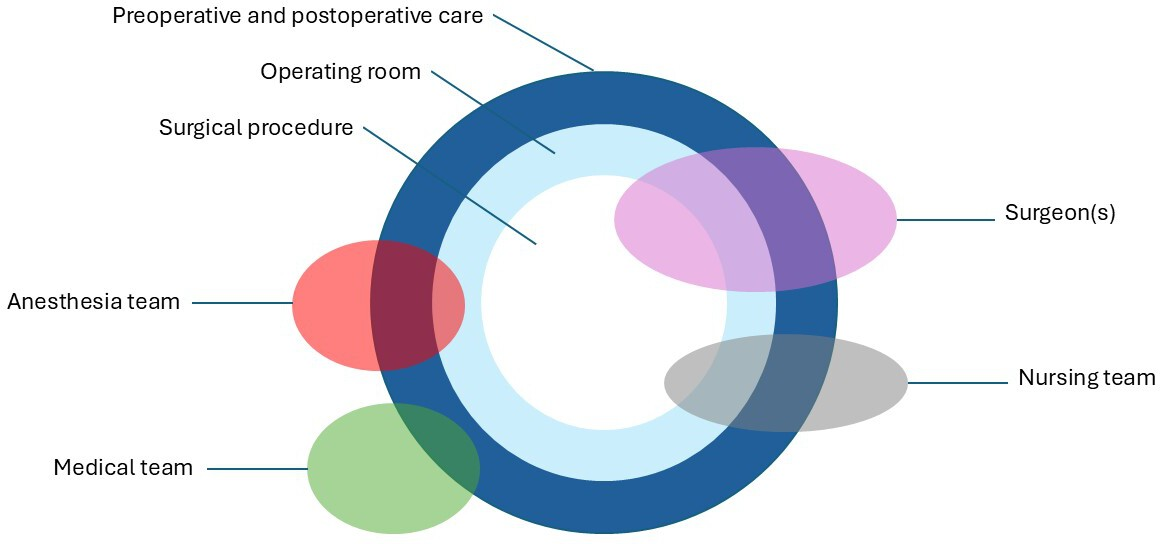
This highlights some key differences between surgical innovation and traditional research. Innovation prioritizes immediate patient care rather than creating broadly applicable knowledge, as with conventional research. For instance, in the case of a young patient with Van Buchem disease causing increased intracranial pressure, surgeons used 3D-printing technology to create a complete skull implant. This life-saving intervention was focused on clinical care rather than research, bypassing typical Human Research Ethics Committee (HREC) protocol approval. In this context, an randomized controlled trials would be unrealistic and unethical, as a control group or sham surgery is neither feasible nor acceptable [23]. Another difficulty in following the randomized controlled trials format is that there are surgical procedures in minimal populations, where it is difficult to have a control group, with results that will not always be statistically significant, and double-blind surgery is impossible [15].
Until over a decade ago, no regulatory framework existed to evaluate innovative surgical procedures unless the surgeon presented them as research protocols. If not, results from non-comparative, non-HREC-approved experimental studies could still be published—unlike those from randomized controlled trials. This flexibility allowed surgeons to introduce complex, untested procedures like laparoscopic cholecystectomy, which, despite success, carried significant risks [24]. The Belmont Report recommended formal protocols for major innovations, but ambiguity around "significant" allowed self-regulation to persist [25]. For example, adult-to-adult live donor liver transplants, which posed high donor risks, initially proceeded without standardized protocols. This gap led some to propose external regulatory mechanisms since procedures not involving new drugs or devices fell outside FDA oversight in the United States [26].
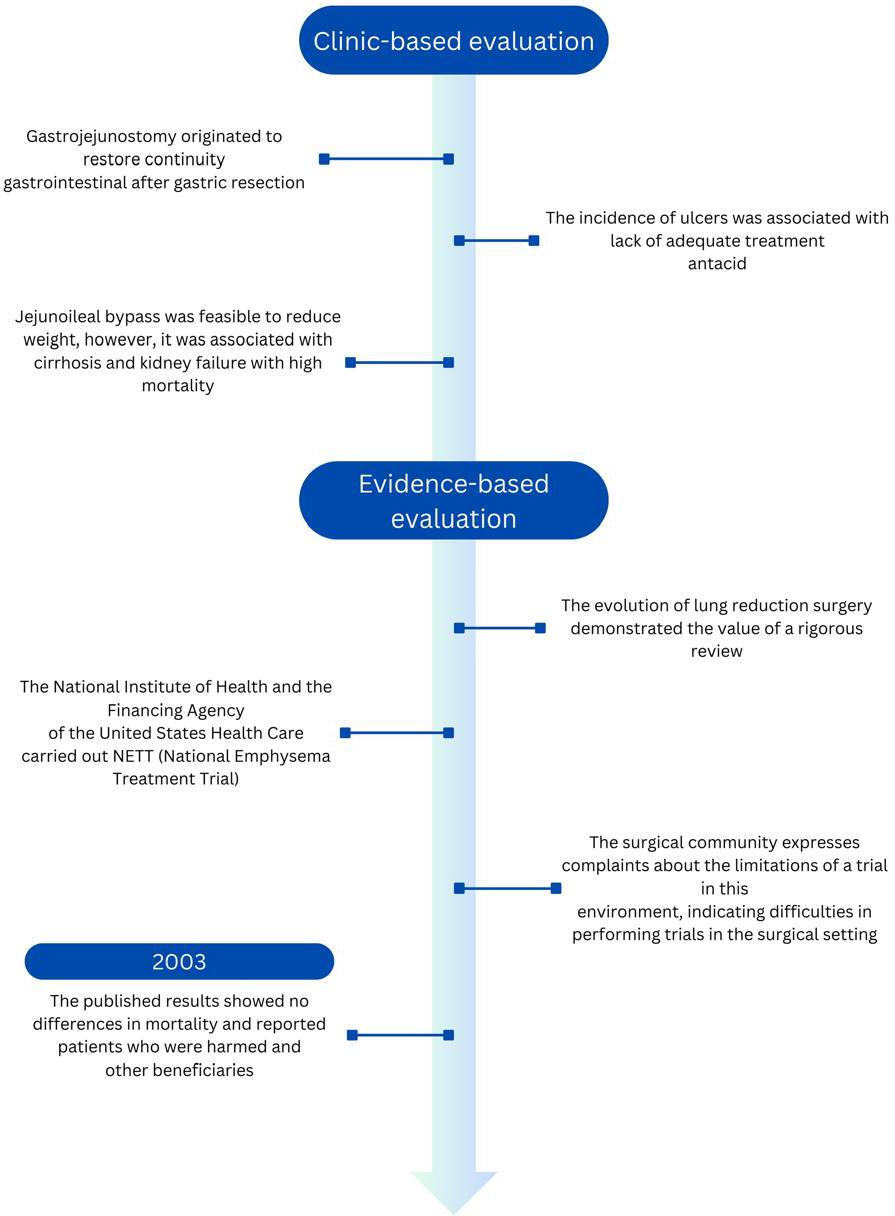
Consequently, in several cases, between innovation and harm to patients was the surgeon’s sense of responsibility, his dedication, and his fear of medical-legal connotations. However, these were insufficient to preserve the safety and effectiveness of these procedures, as occurred with gastrojejunostomy without vagotomy or jejunoileal diversion [27]. In contrast, successful examples of surgical innovations are carried out under exhaustive scientific evaluation, such as lung reduction surgery for emphysema and the development of transluminal endoscopic surgeries through natural orifices in North America (Figure 2). These operations were carried out under the leadership of the Natural Orifice Surgery Consortium for Evaluation and Research (NOSCAR group), organized by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the American Society for Gastrointestinal Endoscopy (ASFE). In 2006, NOSCAR published two papers identifying critical research questions that needed addressing before clinical application. It called for ethical approval and the registration of all human cases, accessible via its website [28].
From the clinic to evidence-based evaluation for innovation in surgery.
Regulations for procedures in surgical innovation: the IDEAL framework
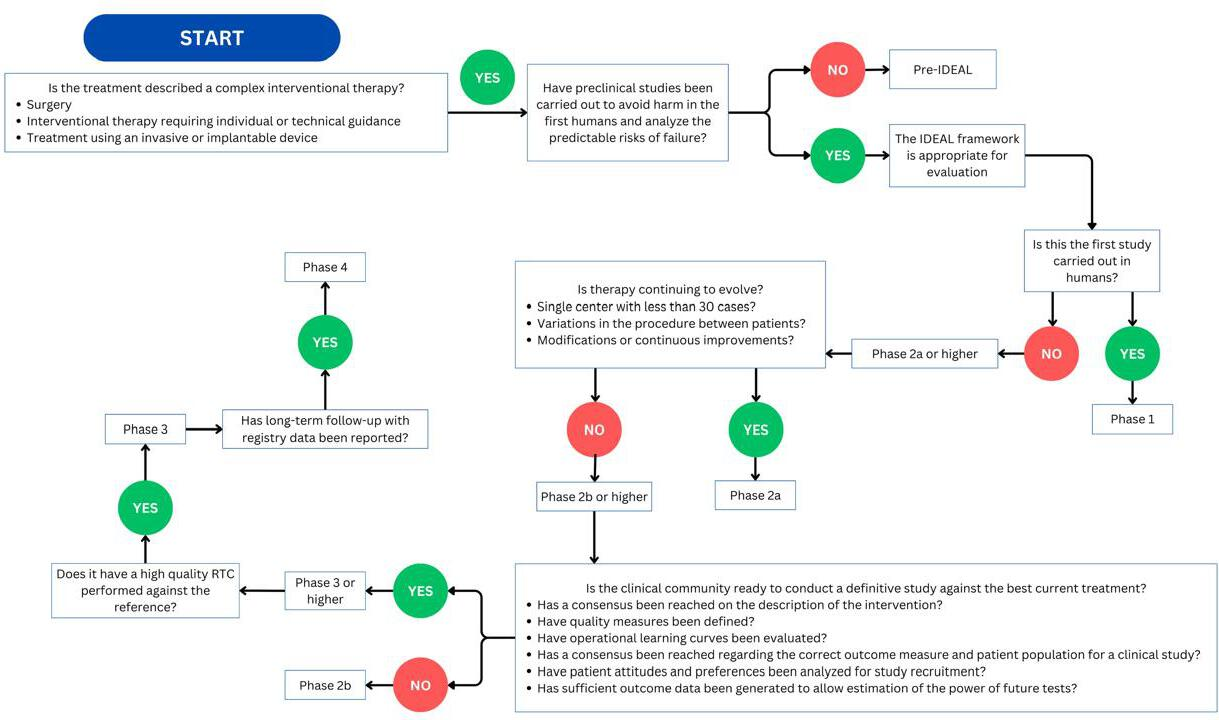
Given the inherent risks associated with innovative surgical procedures, it is essential to establish guidelines that prioritize patient safety. The IDEAL collaboration was formed following a series of meetings between surgeons and methodologists in Oxford to address the challenges of conducting high-quality studies in this field. This initiative seeks to identify the barriers to producing robust scientific evidence and to explore ways to enhance the quality of evidence in surgical innovation [17,18]. IDEAL is a descriptive framework outlining the stages of surgical innovation, starting with an Idea or Innovation, progressing through the Development and Exploration phases, followed by Assessment or Evaluation, and concluding with Long-term studies (Figure 3). This framework offers recommendations for methodology, reporting, and applicable regulations at each stage, as illustrated in Table 1.
IDEAL phase flowchart. Determining the IDEAL phase of a report.
Ethical aspects of surgical innovation: surveillance, consent, learning, and vulnerability
Surveillance
The IDEAL Collaboration developed a framework for surgical innovation, which describes 5 phases of development. In the first phase, when a new surgical procedure is attempted, the innovator should have informed the hospital of his prospective plans, but no HREC approval would be necessary. Then, in the development phase, before the procedure is tested in a small group of patients, approval must be obtained from an HREC to evaluate its effectiveness [29]. Some authors suggest forming an Innovation Committee to manage this type of innovation. However, several opinions remain regarding the format and functions of said committee [16,30,31].
Morreim et al. mentioned that the innovation committe should be in institutions where innovation processes are carried out and comprise members of said institutions with the necessary expertise. The innovation committe had to analyze various aspects, including the criteria for patient selection and managing the surgeons' learning curve [30]. In addition, it had to retrospectively analyze whether realities contrasted with expectations and whether additional innovation studies were required.
Three types of innovation are recognized: minor modifications to standard procedures, significant alterations to established techniques or radical innovations, and innovations new to an institution but validated in other centers. Each type would necessitate different levels of oversight. For the first type, some authors recommend that surveillance is not necessary for certain forms of surgery with minor modifications [32]. Others mention that surveillance is needed, that it should be carried out by peers, a group of interested surgeons, or the head of the Surgery Department in conjunction with an HREC, or even that the HREC alone should carry out the surveillance [31,33,34,35]. A consensus has been reached that a formal review is required for the second type. It can be carried out by an HREC after expert peers and the head of the Surgery Department or a professional external to the institution has presented the procedure. Other authors even recommend a review by a national surgical committee [16,33]. Finally, regarding the last category, various positions have been suggested, ranging from consultation with the head of the Surgery Department to peer review, approval of an HREC, and establishment of an randomized controlled trial 14 [36].
Informed consent
The recommendations vary from how informed consent should be obtained to the type of information to be provided for patients undergoing innovative procedures. About the latter, for example, the following elements are specified: the creative nature of the procedure, a section that describes the learning curve (experience) of the surgeon, the risks and benefits of the procedure, unforeseen or unknown risks, and alternatives to the innovative procedure [16,24,31,35,37,38,39]. Surprisingly, while a slight majority of patients consider the technical details of the operation to be essential information for deciding before an innovative operation, only 20% of surgeons think this should be so. Several suggestions are made regarding the consent format, including a third person (when the treating physician is the researcher or as an aid to the explanation) [40]. A multimedia presentation could be used to explain the procedure to the patient [41].
Learning curve
Likewise, several approaches have been published to monitor this segment. Most of these manuscripts consider that there should be some training for surgeons performing new surgical procedures. Some are in favor of Hands-on training (in animals or human cadavers) [40], others are inclined towards internships with surgeons who already perform this procedure [33] and a third group is in favor of having a mentor or a committee for this type of training [38,39]. The authors also agree that the experience and results should be shared among colleagues [31,38]. A fourth group proposes more structured training for the execution of new procedures, which would imply the introduction of the latest surgical technique so the surgeons would be trained, accredited, and monitored [40,42].
Vulnerable populations
Some authors recommend innovative procedures for vulnerable populations [33,37]. In biomedical research, vulnerability is associated with a heightened risk of harm or exploitation for certain individuals or social groups and a reduced capacity to protect their interests [43]. Factors that increase vulnerability include:
-
Physiological condition: fetuses, neonates, minors, pregnant women, older adults.
-
Pathological conditions: individuals with sensory disabilities, those at the end of life, people with cognitive impairments or psychiatric disorders, and patients with terminal or incurable illnesses.
-
Spatial conditions: prisoners and people held in seminaries or boarding schools.
-
Gender: Women.
-
Cultural and ideological conditions: ethnic minorities and individuals with limited literacy.
-
Social conditions: unemployed individuals, the homeless, and refugees.
There is a need for alternative approaches to informed consent for vulnerable patients [44]. For instance, in emergencies or when treating unconscious patients, some suggest obtaining preemptive exemptions from a Human Research Ethics Committee (HREC) when feasible before performing an innovative procedure [44,45]. In emergency scenarios, consent should ideally be obtained from a family member or guardian. However, there are exceptional cases where proceeding without consent may be necessary and justifiable [44]. In pediatric innovative procedures, informed consent must involve the child’s parents and the patient, where appropriate [46].
The continuous advancement in surgical practice highlights the need for increased research and structured data collection. This will enhance surgical practices by leveraging available data for better outcomes. Essential qualities for fostering growth in surgical innovation include self-reflection, an understanding of vulnerability, and a commitment to change. These characteristics support the cultural evolution needed to build a learning-based approach in surgical practice, thus fostering the development of innovative methods.
Ethical aspects in the stages of the IDEAL framework for surgical innovation
Analyzing how the IDEAL framework minimizes risk in surgical innovations is crucial, as it provides a structured evaluation through each of its five defining phases. The framework’s phased approach is designed to systematically address risks, ensuring thorough evaluation at each innovation process step.
While many researchers and sponsors have recognized the IDEAL framework’s benefits, its adoption internationally has remained somewhat limited. Many research surgeons have cited and applied the study designs and reporting formats recommended by IDEAL, demonstrating growing interest. Nevertheless, researchers expressed a need for more detailed guidance on using these recommendations, especially regarding methodology and ethical research considerations, initially outlined in broad terms [20,29].
Rogers et al. analyzed ethical issues related to harm, autonomy, justice, and conflicts of interest [47]. Then, in 2018, the IDEAL framework explicitly incorporated ethical guidelines for the first time, grounded in well-established research findings and addressing previous gaps. This update marked a safer path forward for surgical innovation by aligning ethical standards with practical guidelines (Table 2) [47,48].
These updated strategies enhance ethical practices in surgical innovation by minimizing patient harm, supporting informed decision-making, ensuring fair and equitable access to innovative procedures, and effectively identifying and managing conflicts of interest.
Conclusions
Surgery, like all medicine, is an applied science, and its progress relies on research and innovation that inherently involve human experimentation. While beneficial, advancements in complex medical technologies for diagnosing and treating diseases are a major contributor to rising healthcare costs. Over the past century, surgical practice has evolved significantly due to advances in knowledge, applications, and innovative breakthroughs in scientific and technological fields, including the inevitable financial aspects associated with surgical care.
Medical education, especially in medical schools and hospitals, serves as the foundation where future professionals are shaped and the origin of the behaviors that will guide their careers. Physicians and surgeons are role models, observed by patients and their families and students. Whether explicitly hired to teach or not, every practitioner imparts knowledge. Professors and mentors become ideals, reference points, and aspirations for students, and they must foster relationships of mutual respect, shared learning, honesty, and collaboration. A teacher’s influence is profound, often distinguishing between successful and disillusioned professionals. The harm caused by a poor teacher is often irreversible, affecting learning outcomes and inflicting various psychological damages.
The responsibilities of surgeons and multidisciplinary teams toward their patients stem not from the profession’s ideology, history, or sociology, nor should they depend on the nature of their compensation. Rather, these obligations arise from the impact of disease on human behavior, the patient’s vulnerability and need for protection, and the intrinsic nature of their relationship with the surgeon and team. This context highlights the need for medical academies, faculties, and schools to regulate and standardize surgical training critically. New surgeons must be educated, emphasizing scientific progress and objectivity, maintaining a balance between bioethics, humanism, and surgical innovation. Achieving this balance will ensure that future generations possess the necessary skills while prioritizing bioethical principles as core elements of surgical education. Moral values should be instilled from the outset to enhance their compassion and humanistic approach in daily practice.
Technological advancements in surgery continually reshape our understanding of diseases, altering the ethical, social, and legal frameworks that govern them. Progress and innovation in surgical procedures underscore the role of bioethics, with patient rights as a central concern. Fundamental bioethical principles for research and innovation in surgery include respect for individuals, beneficence (maximizing benefits and minimizing harm), and justice, which entails promoting good, avoiding harm, and rectifying any damage caused.

