Análisis
← vista completaPublicado el 26 de octubre de 2020 | http://doi.org/10.5867/medwave.2020.09.8051
Identificación y análisis de los ensayos clínicos intervencionales planificados y registrados sobre COVID-19
Identification and analysis of ongoing registered clinical intervention trials on COVID-19
Abstract
Introduction The World Health Organization declared the disease caused by the novel coronavirus (SARS-CoV-2), a pandemic on March 11, 2020. Several studies have been proposed and started since then, mainly covering prevention, diagnosis, management, and treatment.
Objective To identify and categorize all intervention studies up to the end of May related to SARS-CoV-2 infection, according to population and geo-graphical location (emphasis in Latin America) and to verify if there is any correlation according to purpose, phase, and recruitment status.
Methods One thousand six hundred seventy-two trials were selected from 1705 until May 24 on the World Health Organization clinical trials platform related to COVID-19. Jupyter and Python tools were used for data processing and cleaning.
Results One thousand six hundred seventy-two intervention studies related to SARS-CoV-2 infection were found. China, The United States, Iran, France, and Spain are the countries participating in the largest number of studies, while only 4,1% are from Latin America (mostly Brazilian). 28 studies are focusing only on older adults, and ten studies are based exclusively on populations under 19 years of age.
Conclusion The worldwide interest in this new disease is reflected in the increasing number of intervention studies that are being carried out to date. How-ever, the studies analyzed do not cover the most vulnerable age groups proportionally and do not have equitable participation of all the coun-tries. In Latin America, this problem is exacerbated by the region's social, economic, and political limitations. Because it is an emerging disease, there is still not enough information to establish strong correlations between the analyzed variables, and the standardization of protocols is not yet definite because most of the studies are in progress.
|
Main messages
|
Introduction
COVID-19, which is caused by the novel coronavirus (SARS-CoV-2), was first detected in December 2019 during an outbreak in Wuhan, China. Since its appearance, the disease has expanded rapidly to all continents[1],[2]. As a result, the World Health Organization (WHO) declared it a pandemic on March 11, 2020[1], having an approximate number of confirmed cases of 200 000 and exceeding 8000 deaths in about 160 countries by that date[2].
SARS-CoV-2 is the second virus pandemic of the 21st century, after the Influenza A (H1N1) pandemic of 2009[1]. Currently, this public health problem has wreaked havoc on the world population, causing many deaths and adding to the economic, political, and social challenges faced by affected countries[2],[3]. Nations have taken different confinement and mitigation measures to protect the population, especially the elderly and those with comorbidities such as arterial hypertension, diabetes, coronary heart disease, immunodeficiencies, and others. Nations have taken these measures to prevent fatal outcomes and the collapse of health systems[1],[4].
Research has become a fundamental ally for the adequate control and understanding of the pathogenesis of diseases, as well as for the formulation of new therapeutic strategies[3],[5],[6],. The current context has not been the exception since multiple studies related to this new disease are being carried out, covering topics from the diagnosis, management, and treatment of SARS-CoV-2 infection, to strategies to prevent its transmission[5],[6].
Despite the importance of research and intervention studies during this pandemic, there are differences regarding research protocols and access. Factors such as social, environmental, political, and even economic conditions play a preponderant role when investigating[1].
Furthermore, the exclusion of populations such as the elderly and children continue to be pervasive[7], so it is crucial to make their differences visible and demonstrate the impact that new studies have on them[2]. As some authors indicate, these subgroups' particular characteristics must be taken into consideration when investigating since, because of them, the impact of the disease, its prevention, and treatment differ from the general population[7]. The disease caused by SARS-CoV-2 has not been an exception. For example, in older adults, it tends to have more severe manifestations[8], while in children, the symptoms are mild, although a severe manifestation of the inflammatory response has been described[9]. That is why, in this article, we specifically analyze the studies related to these populations.
Similarly, there are geographical regions that historically contribute few studies to the total world scientific literature, as is Latin America, which carries out approximately 2% of the world's research[10]. We studied whether this trend is maintained in this disease.
The main objective of this article is to identify and categorize the intervention studies up to the end of May related to SARS-CoV-2 infection, according to age group and geographic location (with emphasis on Latin America), in addition to correlating them according to objective, phase, and status of their recruitment.
Methods
We did a non-experimental, cross-sectional, and descriptive study. The “International Clinical Trials Registry Platform” (ICTRP) is a network that maintains prospective trial registries. It has a forum from which information is exchanged, and best practices for the clinical trial registry are established. It is made up of primary, partner, and data provider records. This platform includes the studies registered in clinicaltrials.gov, Australian New Zealand Clinical Trials Registry, Brazilian Clinical Trials Registry, Chinese Clinical Trial Registry, Clinical Research Information Service, Republic of Korea, Clinical Trials Registry - India, Cuban Public Registry of Clinical Trials, EU Clinical Trials Register, German Clinical Trials Register, Iranian Registry of Clinical Trials, ISRCTN, Japan Primary Registries Network, Lebanese Clinical Trials Registry, Thai Clinical Trials Registry, The Netherlands National Trial Register, Pan African Clinical Trial Registry, Peruvian Clinical Trial Registry and Sri Lanka Clinical Trials Registry[11].
All available studies related to COVID-19 up to May 24 were downloaded for free at https://who.int/ictrp/en/. When comparing the intervention studies included in Cochrane and PubMed, the WHO database was corroborated for robustness and completion to reduce exclusion biases of investigations that should be part of the present analysis.
The downloadable database consists of a CSV file, where each one of the studies is presented in tabular form. The variables were categorized into thirty-three columns with the characteristics of the studies, such as participating countries, inclusion criteria, minimum and maximum age of participation, and summary. All those classified as “Intervention, intervention study or intervention clinical trial” were selected from the total number of records found.
The data was standardized since many columns had combined numerical and nominal formats. We used the free virtual tool for data processing Jupyter and Python programming language to carry out an adequate purification and transformation of the data obtained to generate a statistical model for its visualization. For the correlation matrices, the ScyPy library and Pearson's correlation coefficient were used. Statistical significance was established to be greater than 0.5.
For adequate analysis of the information, the following variables were taken into account: study phase (ordinal), recruitment status (nominal), the purpose of the study (nominal), participating countries (nominal), and minimum and maximum age (both type variables discreet). These were chosen because their tabulation allowed an adequate classification for statistical analysis. The following variables were excluded: trial ID, last update, available title, scientific title, acronym, sponsor, date of the first record, date of the second record, source of record, web page, condition, intervention, primary outcome, expected date of results, the actual date of results, link of results, bridging flag, bridged type, and the existence of results. Since not all the studies had such information, they were not suitable for the present study's objective and were not statistically assessable.
Results
Up to May 24, 2020, 1 705 studies fulfilled the required characteristics for the investigation. However, in a comprehensive review, it was possible to identify that according to the title and characteristics of the studies, four of them were not related to COVID-19, and another 29 were misclassified (the abstract described the study as observational), so we decided to exclude them from the present analysis, leaving a total of 1 672 articles.
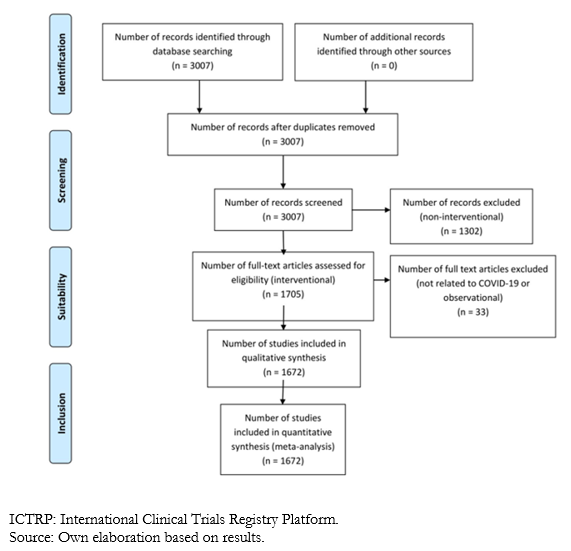 Full size
Full size As noted in table 1, the countries that contribute the most studies are China (364), United States (226), Iran (171), France (128), and Spain (111). The rest contributed less than 100 studies. Latin-American countries participated in only 81 clinical trials. Brazil participated in 30 studies, Mexico in 17, Argentina and Colombia in nine, Cuba in five, Peru in four, Chile in three, and Honduras, French Guyana, and Ecuador in one each. Notably, Argentina y Brazil participated jointly in 1 clinical trial.
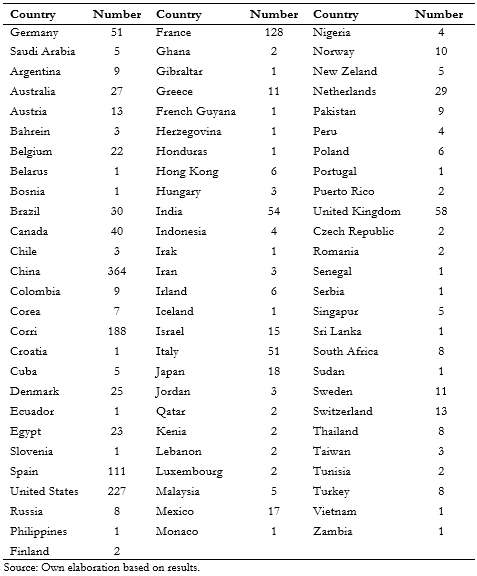 Full size
Full size The countries with more trials without age limit are China (249; 63% of total), Iran (84; 44%), United States (59; 26%), Spain (42; 37%), France (26; 20%) and India (17; 31%).
Only five countries in Latin-America registered investigations in which the inclusion criteria did not establish an age limit; they were distributed as follows: Mexico seven, Columbia four, Brazil three, Argentina one, and Cuba one. Of these studies, three are currently in phase two, five studies in phase five, one study in phase one, and the rest did not report this parameter. Eleven clinical trials sought to research treatment, and the rest were classified as others. It should be noted that, of the 13 clinical trials, 11 were conducted by individual countries, while two were multinational and included Mexico.
On the other hand, 694 studies have an established maximum age, and their distribution can be seen in Figure 2. Some are from the pediatric population, so the maximum age ranges from seven years to 130 years. The average maximum age is 74.7 years, with a median of 75.
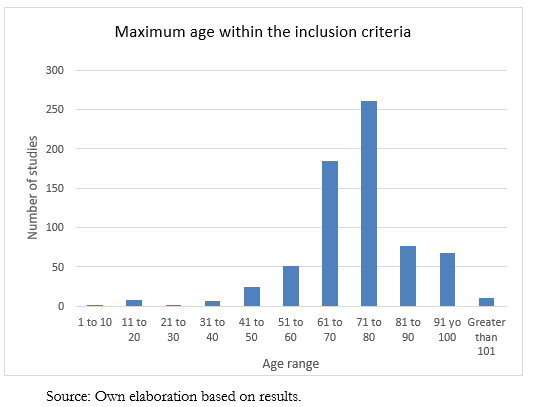 Full size
Full size There was no significant relationship between the established cut-off age and the variables of the study phase, countries, or genders of inclusion. Table 2 shows the information obtained according to phase, recruitment status, and purpose. Of the total studies analyzed, 36.8% do not have information about the stage. On the other hand, in phase two, 21.6% of them are registered, while in phase three, there are 16.9%. The remaining, which comprise 22.4%, are distributed between phases zero to four. When analyzing the recruitment status, 799 are recruiting, while 723 have already completed this process.
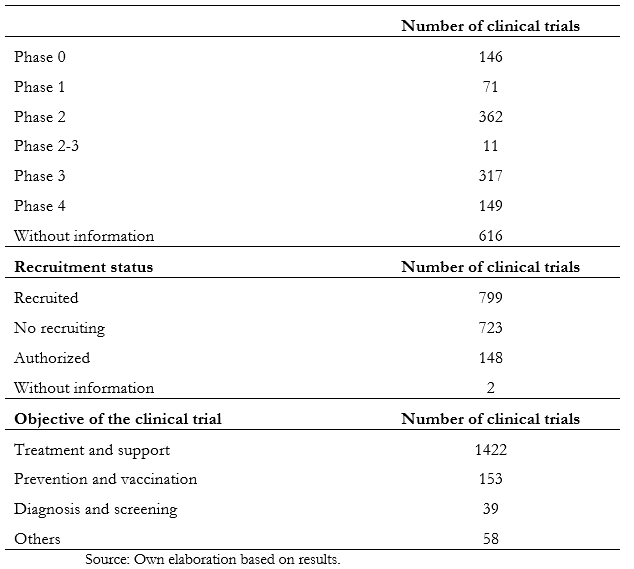 Full size
Full size Table 3 only describes studies that involve Latin American countries.
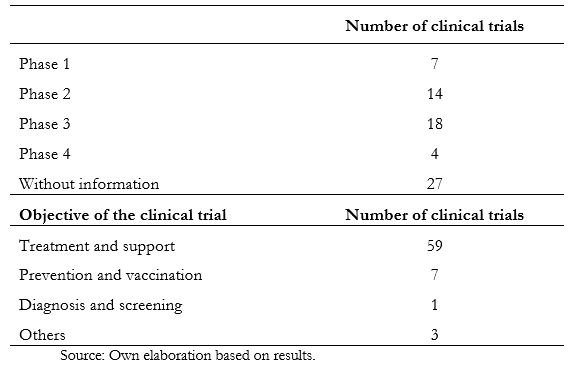 Full size
Full size Elderly population
Among the studies analyzed, 1 581 articles include the elderly population. Of those studies, 896 reports no age limit, while 694 have a maximum age greater than 65. Of the studies that do not establish an age limit, 105 have this age group's participation, and nine excluded them. The rest do not have such information.
Of all studies, 28 focused on including only the population over 60 years of age, equivalent to 1.6% of clinical trials. One of them is taking place in Latin America, specifically in Cuba. Of these studies, 16 are aimed at research in treatment and support, 11 are for prevention, and one was classified as "other."
Pediatric population
A total of ten investigations exclusively include the population under 19 years of age. They were carried out in France, China, the United States, Ghana, and Canada. Three are for treatment purposes, two for prevention, one for diagnosis, and the rest are unspecified. A significant relationship between the established age limit and the variables phase, countries, or inclusion gender was not found.
One hundred thirty-six studies have as inclusion criteria an age under 19 years, including the adult, pediatric, and adolescent populations. Thirty-six studies do not establish a minimum age in the inclusion criteria. Thirty-two do not have this information.
Of all the studies that include a population under 18 years of age, 81 are for treatment, five for prevention, three for diagnosis, four “other,” and the rest do not indicate it. There are three studies in phase one, 23 in phase two, 44 in phase three, and five in phase four. There are 20 in phase zero, and the rest do not indicate it.
The countries participating in these studies are Iran in 42, China in 41, India in 16, the United Kingdom in 14, the United States in nine, Egypt and France in seven, Italy in six, Canada and Japan in five, Mexico and Sweden in four, Belgium, Germany, Korea, the Netherlands, Spain, and Switzerland in three, while Brazil, Hong Kong, Pakistan, Singapore, and Taiwan at two each. Chile, Denmark, French Guiana, Ghana, Malaysia, New Zealand, Norway, Senegal, Sudan, and Thailand participated in one study.
Discussion
According to multiple authors, experimental studies constitute the ideal research methodology and are the gold standard for evaluating both non-pharmacological interventions and the safety and effectiveness of a drug in a population[12],[13]. Therefore, it is vital to categorize experimental studies investigations, even more so when dealing with a pandemic pathology[3].
During data collection, it was possible to determine that intervention studies have been published in a total of 78 countries. Fourteen belong to the American continent, 23 to Asia, 32 to Europe, eight to Africa, and one to Oceania. According to the results obtained, China, the United States, and Iran have the most clinical trials of this type. China has the highest number of registered studies, being the first country to be affected by the disease and, for several months, until March 2020, remained as the site with most cases worldwide[2],[13],[14]. By mid-February in some countries, COVID-19 cases had not yet been registered, while China already had an accumulated experience of almost three months and more than seventy thousand patients, so clinical investigations are more abundant in this nation[2],[13],[14]. On the other hand, the United States has historically led the research, and in 2017 it had published the most on global outbreaks[15]. Moreover, the U.S. displaced the others in the number of cases worldwide, occupying the first place until May of this year, with more than one and a half million cases and a mortality rate of 5.8 percent[14].
There has been a significant increase in the number of published articles in Iran during the last 15 years, mainly from medical literature. It has one of the fastest growth rates of scientific productions globally, with a considerable increase in health[16].
As COVID-19 is a novel pathology of recent origin, the investigation speed in the studies' different clinical phases has not been enough to reach the end of most studies. Until now, five months after the onset of the pandemic, only 8.9 percent of the trials are in phase four. The same happens with the recruitment status, where it is evidenced that less than half (47.7%) are recruiting a population sample.
Regarding the study's purpose, 85 percent of them are related to treatment and support of the disease, and the same trend is maintained if studied by regions—as is the case in Latin America. This is important since there is no approved and effective treatment against SARS-CoV-2 infection, so research aimed at recovering patients is essential[17].
The situation in Latin America
Latin America participates in only 3.5 percent of the studies analyzed. Of these, 84.2 percent aim to investigate the treatment and support of COVID-19; ten percent focus on prevention, 1.4 percent on diagnosis and screening, and 4.2 percent on other issues. The majority (25%) are in phase three, 15 percent in phases one and two, and 5.7 percent in phase four.
Based on the data obtained, Brazil contributes the most to COVID-19 studies with 42.2 percent, while Mexico and Argentina contribute 23.9 percent and 12.8 percent, respectively. This coincides with the region's trend, in which classically the countries that carry out more studies are those with the largest population, such as Argentina, Brazil, and Mexico, and followed by Colombia, Peru, and Chile[16].
By the end of March of this year, the number of cases related to the COVID-19 disease increased significantly in Latin America[14], so research in the area should be mandatory. However, historically this region generates a lower amount of research compared to others[15], a trend that was reproduced when performing this analysis. Consequently, articles from Latin American countries are underrepresented in the leading specialized journals due to different barriers, for example, little institutional interest, few incentives, little investment in research, political issues, among others[18].
Latin America's problems include difficult sanitary conditions and economic, social, and political problems that may underestimate Brazil and Nicaragua's caseload, for example. Those factors could result in untoward outcomes regarding the spread of the disease[1].
These regional characteristics predispose to new challenges regarding the spread of the virus in this region. Hence, the importance of generating research that can benefit the most affected population[1].[5].
Latin America could learn from external experiences to avoid reaching European countries' dramatic situation during the first wave of the current pandemic. Although health measures have already been followed to prevent the massive spread of the virus and the collapse of health services, conducting studies is an important measure that allows the generation of knowledge necessary to optimize decisions in the management of the pandemic[1],[2],[5].
Elderly population
According to some series, intervention clinical trials that exclusively include older adults have been reported to be few, between one and two percent. They are generally studies with small sample sizes, even for diseases prevalent in this population[2].
Clinical trials try to control as many variables as possible, and older adults have many comorbidities and pathologies that can alter their results[7]. COVID-19 is no exception. Although most studies (94%) could include older adults (since they are established without an age limit or the limit is greater than 65 years), only 1.6 percent of them analyze those older than 60 years as a subgroup, which may result in an underestimation of the consequences of both the disease and its treatment in this age group[19].
Most of the clinical trials analyzed are related to the disease's prevention and treatment, which are fundamental objectives since this population has higher mortality from COVID-19[8]. It is widely known that pharmacokinetics and pharmacodynamics change substantially after 75 years[2]. For this reason, the risk and benefit of new treatments in the management of COVID-19 should be studied extensively, and more research should be established with the primary purpose of determining efficacy and safety in this subgroup.
Regarding the geographical distribution of clinical trials that include the elderly population, most of them are carried out in China, the first country affected by COVID-19. They are also carried out in regions where until May 2020, there were many cases, for example, the United States and Spain[14]. More studies of this subgroup are scarce in all regions of the world, and few were found in Latin America. For example, until mid-May, the country with the most confirmed cases in the region was Brazil[14]. However, despite having well-established ethical and regulatory standards in research, Brazil does few clinical trials involving older adults. Some authors have suggested that the delay between the approval time and the commencement of clinical trials in this country can be a limitation[8].
The older adult population has, in general, a similar risk of infection with the SARS-CoV-2 virus. However, many of them present comorbidities such as diabetes, arterial hypertension, cardiovascular disease, or cerebrovascular disease, and these, added to age, do confer a higher risk of fatality. According to some hypotheses, this is due to weakening the immune system associated with other diseases[8].
The elderly population has the highest mortality associated with COVID-19 disease because they are more susceptible to becoming seriously ill but less likely to be admitted to an intensive care unit[18]. It is described that the elderly population is more prone to get pneumonia-complicated COVID-19 and multisystemic failure. These other complications should be prevented when approaching an elderly patient[8].
Pediatric population
Of all studies analyzed, only 0.5 percent of them focused exclusively on the population under 19 years. Of these, 30% targeted COVID-19 treatment, 20% prevention, 10% diagnosis, and 40% did not provide information on the research purpose.
As is already known, the epidemiology of COVID-19 differs significantly between different age groups. It has been shown that the incidence in children is significantly lower than in the general population, which can vary between one and two percent[20],[21]. Lower exposure of the pediatric population to the environment can explain this difference[22].
The lower incidence of this disease in the pediatric population may be why few studies focus on this subgroup. However, although the number of cases in children is lower, they are considered an important source of contagion[9],[15], since the majority present as mild or asymptomatic cases[9]. Therefore, including this population in diagnostic studies is of great importance to obtain real data from a probably underdiagnosed[9].
Despite that the most frequent clinical presentation is mild or asymptomatic, an association has emerged between Kawasaki disease and COVID-19 in children, specifically in those with a robust inflammatory response, which is generally severe or fatal[23],[24]. Given this emerging syndrome, more research should be done to address these patients' possible treatment and their evolution.
Of the total number of studies that include the population under 18 years of age (136), 30% did not indicate which phase they are in, 32.3% are in phase three, 16.9% in phase two, and the remaining 20.5% are distributed between phases zero, one, and four.
All the investigations that focus on this subgroup are carried out in countries with a high human development index[1], except for one study carried out in Ghana. It should be noted that none of them includes Latin American nations[1]. Despite the Latin American fertility rate decreasing in recent years, it is significantly higher in the poorest countries of the region (up to five children per woman)[25], so it can be extrapolated and consider a higher pediatric population density in these countries. Consequently, it is important to generate studies in the pediatric population in the region.
Although vertical transmission has not been documented as a form of contagion, newborns of infected mothers have been documented COVID-19 positive, even at 36 hours of age[26]. In the studies analyzed that included the pediatric population, 26.4% did not establish a lower age limit. However, none of them have been exclusively dedicated to preventing, diagnosing, managing, or treating COVID-19 in the neonatal population. This may be a precedent for future studies to be carried out in Latin America exclusively focused on the spread of SARS-CoV-2 infection in neonates.
Limitations of the study and future considerations
A possible bias is that an arbitrary time cut-off for the inclusion of the clinical trials was chosen. New clinical trials and information come up every minute. Fundamental studies could be involuntarily excluded in the course of this pandemic. This type of study must be reproduced in the future to guide the scientific community about information deficits in the research necessary for an adequate approach to the pandemic.
Although a robust database was used and confirmed that it had the studies registered in PubMed and Cochrane, it cannot be guaranteed this database to be completely exhaustive.
During data collection, the variables aligned according to the study's objectives were selected, while those that did not have a relationship with the study or could not be statically significant were eliminated. Thus, new studies could be generated in the future where information that was not taken into account for this writing could be included.
It is essential to highlight that research and the permissibility of conducting clinical trials is not the norm in all regions or countries, so errors can be generated when standardizing management and care protocols based on studies carried out in geographic areas with different sociodemographic characteristics. Also, there may be recruitment bias in the different clinical trials, where people who belong to ethnic minorities, as well as those who do not have access to health services or are affected by other economic and social factors, geographic and cultural, are underestimated in these studies[27].
Few studies include special populations, such as children, elderly, pregnant patients, lactating, or neonates, so it is important to include clinical trials that are open to these subgroups or specific for them because their characteristics differ from the general population. Regarding older adults, there is still no information necessary to know what the real maximum age will be, and new studies will have to be carried out to determine if the percentage of participation will be statistically significant.
Conclusions
Through the recompilation and categorization of the intervention clinical trials related to COVID-19, it was documented that few months after the onset of the pandemic, there was an increased worldwide interest for research of the infection, driven by the rapid growth of the disease, the high rate of contagion, and its global expansion.
The countries that traditionally have a more significant research track record have also led the number of intervention clinical trials related to COVID-19 up to May 2020. In addition, nations that have suffered a more significant impact from the SARS-CoV-2 infection have provided a larger number of studies about this topic.
In Latin America's specific case, countries that registered more COVID-19 investigations have a larger population. Nonetheless, clinical trials in this region are scarce and scattered, representing a low percent globally. Furthermore, there is a relationship between social, political, and economic limitations and the conduct of investigations during this pandemic.
The number of clinical trials is not evenly distributed concerning special populations such as children and older adults. There are few unique studies for these age groups; additionally, the general population's results cannot be extrapolated because of their particular physiological characteristics.
Because COVID-19 is an emerging disease, many studies are still ongoing, so establishing definitive conclusions and protocols based on evidence-based medicine and per research results is premature. Further epidemiological and statistical analyses of the disease should be performed.
Notes
Roles and contributions of authorship
LSS: conceptualization, formal analysis, data curation, research, methodology, administration, resources, writing, and review. GHW, MCV: discussion, data curation, research, methodology, administration, resources, writing, and review.
Conflict of interest
The authors declare not to have any conflict of interest.
Financing
This article was self-financed.
Declaratory of date availability
The authors declare that the data obtained from this research are available upon request.
From the editors
The original version of this manuscript was submitted in Spanish. This English version was submitted by the authors and has been lightly copyedited by the Journal.

