Análisis crítico
← vista completaPublicado el 15 de enero de 2016 | http://doi.org/10.5867/medwave.2016.01.6365
Revisión de las guías actuales de fibrilación auricular
Current clinical practice guidelines in atrial fibrillation: a review
Abstract
The aim of this study is the methodological evaluation of Clinical Practice Guidelines (CPG) in atrial fibrillation. This is the second in a series of articles of review, analysis, assessment in methodology and content of clinical practice guidelines in Cardiology. Among all clinical practice guidelines, we selected the American, Canadian and NICE (National Institute for Health and Care Excellence) guidelines. We used the AGREE (Appraisal of Guidelines for Research and Evaluation) II instrument for the assessment. In general, the guidelines obtained the lowest score in the applicability domain (mean 36.1%); while the highest score was for clarity of presentation (mean 93.5%). The lowest percentage was found in the editorial independence domain (Canadian guideline) and the highest of all scores in the applicability domain (NICE guideline). Regarding global quality, the NICE guideline obtained the AGREE II instrument best scores, followed by the American guideline, both recommended for use without modifications.
Introduction
Atrial fibrillation (AF) develops when there is a structural or electrophysiological abnormality altering the atrial tissue and promoting an abnormal formation of the electrical impulse of the heart and its conduction. It can be caused by various mechanisms, representing the final phenotype of multiple diseases [1], being considered the most common arrhythmia in clinical practice [2]. Patients with atrial fibrillation have 5-7 fold increased risk of cerebrovascular accident (CVA) than the general population, there is a phenomenon of increased number of patients with atrial fibrillation worldwide [3],[4]. The relevance of atrial fibrillation is high because even among low-coronary risk individuals without clinically significant cardiovascular disease, atrial fibrillation is still associated with an increased mortality [3],[4].
The diagnosis and management of chronic diseases such as atrial fibrillation is currently based on the use of Evidence Based Medicine (EBM) [5], which provides guidelines for clinical decision making through acknowledging the statistical probabilities of their statements, and prioritizing the information obtained. In one of the highest levels of the pyramid stand clinical practice guidelines [6], collections of recommendations of the highest level of scientific evidence which aim to help clinicians and patients to make an informed decision for specific clinical circumstances [5],[7]. The origin of a clinical practice guideline is a systematic review of studies conducted by a clinical question, considering the higher level of evidence to make an informed clinical decision. The clinical practice guideline recommendations lead the management of patients with a specific clinical condition, and involve testing and value judgments, based on the benefits and risks of alternative care options [8].
Cardiology is no doubt one of the medicine specialties that has greater scientific production and development of clinical practice guidelines according to EBM; given the high prevalence of chronic diseases such as hypertension, atrial fibrillation, heart failure, and etcetera. We assessed clinical practice guidelines quality by means of the AGREE II instrument, which through a checklist, evaluates the minimum criteria that must be present in a clinical practice guideline supported by EBM. This instrument is not only used for external evaluation (authors or institutions outside the development team), but also as an instrument of self-assessment of quality by the development team. The importance of methodological quality of a clinical practice guideline is the possibility of providing relevant and appropriate recommendations [9], thus their evaluation is useful to support its external validity, applicability and clinical relevance [10].
It is important for a clinician the use of these important tools that contain valuable information; but they are dependent on the included studies characteristics for the extrapolation of recommendations to different populations. For example, there are large significant differences in mortality associated with atrial fibrillation between developed and developing countries, probably due to the different distribution of etiologies, comorbid conditions, and management approaches [4]. This could have a significant impact on the treatment of atrial fibrillation. Therefore, it is appropriate for each region to perform its own clinical practice guideline based on studies in the same population, but the development of a guide represents a very high cost for developing countries, and it is easier the adoption/adaptation of an external guideline. To perform this process, it is important to recognize which is the best methodological quality clinical practice guideline, therefore the objective of this study is to conduct a methodological evaluation of the clinical practice guidelines in atrial fibrillation, through AGREE II instrument as in a previous article in this series [11].
Methods
This is the second in a series of review articles, analysis, assessment methodology and content of the clinical practice guidelines in cardiology. A specialist conducted the search of the literature and previous studies [12],[13],[14],[15],[16]. A systematic search of clinical practice guidelines was performed using keywords, generic filters and MeSH terms: atrial fibrillation, practice guidelines, clinical practice guidelines in databases such as National Guideline Clearinghouse, Scottish Intercollegiate Guidelines Network (SIGN), The National Institute for Health and Care Excellence (NICE) and MEDLINE. Between 2013 and 2015 eight guidelines were found for adults, of which the American guideline [1], NICE [17] and the Canadian guideline [18] were selected due to having their last update less than two years before (Table 1). A methodological evaluation was performed using the Appraisal of Guidelines for Research & Evaluation (AGREE II) instrument. Four evaluators conducted an analysis of each guide, and discrepancies were resolved by consensus, as in previous studies [12],[13],[14],[15],[16].
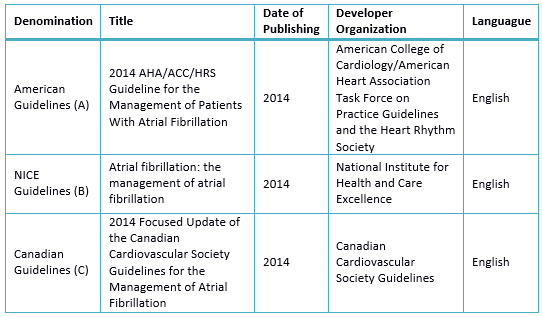
Table 1. Selected Clinical Practice Guidelines
Results
The guidelines have common basic characteristics evaluated (Table 2 and 3), and two clinical practice guidelines have linked its recommendations to the levels of evidence (Table 4).
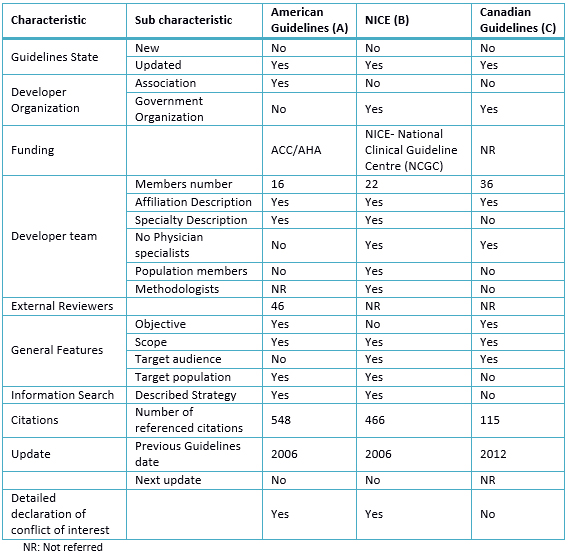
Table 2. Characteristics of selected Clinical Practice Guidelines
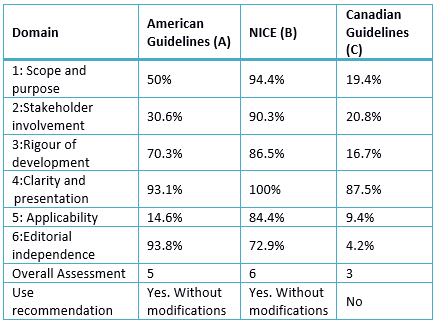
Table 3. Rating Domains (%) for selected guides
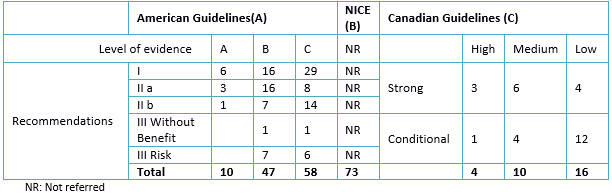
Table 4. Summary of levels of evidence according to GRADE * recommendations
1. Domain 1: scope and objectives
A. 50%: Despite not having a specific paragraph to determine a goal, a general approach is presented in decision-making in the diagnosis, treatment and prevention. The target population is not defined, and the health aspects is the optimal management of atrial fibrillation. The target population for which it is intended to apply is subtly described when different indications of treatment for different population groups with comorbidities are named.
B. 94.4%: Report as objectives to provide management recommendations for health professionals, with a focus on its role in health policy and the patient. Health aspects include risk stratification and best approach. For each aspect, such as education, reference, risk stratification, the clinical practice guideline addresses the type of review (intervention or prognosis), review questions and results. The population is specifically described in terms of age, including types of diseases (various presentations of atrial fibrillation). Further aspects not covered by the guideline are reported.19.4%: No reports of a specific objective item, making a generalized and non-specific description. Health aspects are poorly described and does not report the population it is intended to apply the guideline.
2. Domain 2: stakeholder involvement
A. 30.6%: The names of the members, workplace but not their specialty are reported, and methodologists are not distinguished. It is not specified whether the views of the target population in which the guide could be applied, were included. The guideline target users (type of specialists) is not detailed.
B. 90.3%: The developer group includes individuals from all professional groups: "cardiologists, cardiac nurse, pharmacist, general practitioner, primary care physician, emergency physician, cardioversion and cardiac rehabilitation nurses, a representative of the patients, cardiology and electrophysiology professor, geriatrician, technical crew, researchers, health economist, cardiothoracic surgeon, hematologist ". In the guideline development, the views of patient care with an approach focused on them were considered. The development of recommendations involves patients in treatment decisions and care. In addition, it has advices for health professionals on "help in the patient's decision." The target users of the guideline are health professionals dealing with patients with atrial fibrillation. It describes how the target audience can use the guideline.
C. 20.8%: It describes names, institutions and geographical location, but does not report the role of each of the authors or their specialty. It does not consider the views of the target population, though partially describes the target users (specialists and health personnel).
3. Domain 3: rigor of development
A. 70.3%: The use of systematic methods for the search of evidence, accompanied by the search strategy, databases and keywords are reported. The criteria for selecting the evidence are reported in the supplement, where authors, objectives of each work, sample size, intervention and comparison, inclusion and exclusion criteria, cutoffs, measures of association, adverse effects and limitations are detailed. The strengths and limitations of the body of evidence is not reported, although this information is in the supplement. They present the designs of the included studies, methodological limitations, and relevance of the primary outcome variables, consistency and direction of the studies. Likewise, the process of recommendations developing is presented, although the use of a method to reach consensus is not stated. The recommendations include assessing the evidence in terms of risk or benefit. There is an explicit link between the recommendations and levels of evidence, and each item relates to its reference. A group of official reviewers reviewed the guide; also, the institutions editors approved it prior to its publication; although the description of the review process, used methods or results of the reviewers were not included. A procedure to update the guide is not included.
B.85.6%: A systematic search in Medline, EMBASE, and other databases was performed. The search period, used terms and advanced strategies were detailed. The studies published to October 2013 were considered. The criteria for selecting the evidence were described; the strengths and limitations of all evidence, describing the methods used to formulate the recommendations were considered. These considered the benefits, side effects and costs. There is an explicit link between the recommendations and the evidence supporting it, summarized in tables comparing economic and clinical evidence. The guideline includes a review of six weeks, with a subsequent re-evaluation to determine whether evidence has progressed significantly to alter the recommendations and thus ensure an update.
C. 16.7%: The methods used for the search neither reported the selection criteria, nor the strengths and limitations, nor the methods for formulating the recommendations of evidence. It considers the risks in formulating the recommendations, but it does not categorize the recommendations as a benefit or risk. The recommendations are accompanied by a paragraph of description, but the guideline does not include a review by outside experts. A procedure to update the guide is not included.
4. Domain 4: clarity and presentation
A. 93.1%: even though the purpose or intent is not clearly specified, the recommendations are specific, identifying the relevant population, warnings or qualifications. The various atrial fibrillation -related conditions are clearly presented and recommendations are divided by conditions and / or states.
B. 100%: The recommendations are specific, considering the different management options, with key recommendations easily identifiable.
C. 87.5%: The recommendations are specific and unambiguous, and the various management options are presented, with key recommendations easily identifiable.
5. Domain 5: applicability
A. 14.6%: Factors that favor or impede its application are not described, neither the algorithms, nor tools to take advantage of the guideline. The costs of applying this guideline in the target populations were not considered. It does not offer criteria for audit, if it is implemented in a specific population, nor clinical criteria for monitoring patients in order to assess the impact of their use.
B. 84.4%: It describes facilitator factors, detailing the process by which the guide was developed with tools on how the recommendations will be put into practice. Resources have been addressed using economic studies, and evaluating cost effectiveness. It devises monitoring criteria in the implementation section.
C. 9.4%: does not describe facilitators and barriers to implementation. It includes summary documents, and algorithms, but does not include links to help manuals or instructions for how users can access tools and resources. Not taken into consideration the economic aspects and there are no criteria for monitoring and / or audit.
6. Domain 6: editorial independence
A. 93.8%: the points of view of the funding staff do not represent a bias at the time of guideline preparation, because those with conflicts of interest refused to vote in the segments in which they could see themselves involved. The body of evidence considers studies that were funded by pharmaceutical laboratories, so it is not possible to say that there was not any bias; yet, the conflicts of interest of editors were recorded in detail.
B. 72.9%: The National Center for Clinical Guidelines was commissioned by the National Institute for Health and Excellence Care to do the work in this guideline. The authors reported conflicts of interest, as described in each of the events, and how they have changed through the development of the guideline.
C. 4.2%: There is no explicit statement on the guideline financing. On their website, there is a statement about the financial support for the realization of this guideline, reporting that three pharmaceutical industries contributed. Conflicts of interest of the developer group are not detailed.
7. Global evaluation of the guide
A. 5: recommended use unchanged.
B. 6: recommended use unchanged.
C. 3: use is not recommended.
Discussion
When analyzing the selected guidelines there are some highlights and some limitations within them.
Of the six domains from Appraisal of Guidelines for Research & Evaluation (AGREE II), guidelines obtained the lowest score in the applicability domain with an average of 36.1% (9.4% - 84.4%); while the clarity of presentation domain scored highest with an average of 93.5% (87.5% - 100%). Separately, the lowest score was in the domain editorial independence (Canadian guide) and the highest of the scores was in the applicability domain (NICE guide). The NICE guideline had high scores in the domains and the best overall assessment, with a score 6/7. Regarding the use, application without modification is recommended; compared to the Canadian and American guidelines, the first recommended with modifications and the second not recommended, respectively.
Of the three guidelines studied, the NICE guideline recommendations did not link the levels of evidence to the studies as in the previous version of 2006 [19]. In the American guideline, most of the recommendations are class I level of evidence A. In the Canadian guideline, most recommendations are of low level of evidence with conditional recommendations.
The NICE guidelines as the American guideline were supported by institutions and funded by them. The Canadian guideline has been developed with the support of the Canadian Society of Cardiology, but has not reported its funding. On the update, the American and NICE guidelines were made after a long period (previous guidelines of 2006) as opposed to the Canadian guide, whose previous guideline was made in 2012. This aspect has led to perform a complex procedure for development guidelines which is accompanied by an extensive literature review; the American guideline has 548 references, NICE guideline has 466 and the Canadian guideline has 115; the latter partly due to regular updating. It should be noted that both guides have been updated eight years later, not taking into consideration that a guideline becomes outdated on average two years after their publication [20] and should be reevaluated at least after three years [21].
Among the most important changes present in the guidelines evaluated, the American guideline has given greater importance to the recommendations of antithrombotic therapy, based on the risk of thrombosis, regardless of the type of atrial fibrillation; using the CHA2DS2VASC [22] instead of CHADS [23] (recommendation class IB) to assess the risk of stroke. The NICE guidance also supports risk assessment based on the CHA2DS2VASC, unlike the Canadian guide that maintains the use of CHADS supplemented by the inclusion of some criteria CHA2DS2VASC. These changes are important because the use of these risk scales will lead the management, however for some authors there is no difference in the net profit of these risk scales [24].
Regarding the assessment of bleeding, the American guideline uses the bleeding risk scores HAS-BLED [25] and HEMORR2HAGES [26]; unlike NICE guidelines which recommends the use of HEMORR2HAGES score [26] with moderate evidence and failing to discriminate risk groups; while the Canadian guideline uses only the HAS-BLED score [25]. The NICE guideline development has been based on a patient-centered approach, and included members of the population in the development team. This aspect gives it great merit, because the patients play an important role, since many of them at high risk for atrial fibrillation give more importance to prevent bleeding stroke, opposed to the suggestion made by various clinicians [27].
Regarding the benefit of using aspirin, NICE guideline does not recommend its use for the prevention of stroke, similar to that mentioned by the American guideline that claims aspirin did not reduce stroke in patients over 75 years. Additionally, the American guideline suggests the use of aspirin only when oral anticoagulation is not feasible, although the American College of Chest Physicians Guideline suggests giving these patients clopidogrel and aspirin combined therapy [28]. The benefit of aspirin use has been discussed predominantly based on clinical trial SPAF-1 [29], which showed the benefit of aspirin alone in preventing stroke in patients with atrial fibrillation (19% reduction in dose 325mg); and the BAFTA study [30], which compared warfarin and aspirin in patients at high risk of more than 75 years, reporting that those treated with warfarin had less stroke rates and similar bleeding rates. The following studies have not supported the use of aspirin [14],[16]. It could be summarized that aspirin is recommended for primary prevention of stroke in patients with atrial fibrillation, and vascular procedures such as percutaneous coronary intervention (PCI). In secondary prevention, aspirin is recommended in high-risk patients with coronary artery disease, acute coronary syndrome, not embolic stroke/transient ischemic attack, and peripheral arterial disease to prevent further vascular events, but not in patients at low risk for cardiovascular events; being recommended 75-100 mg / day doses [31].
Regarding anticoagulation, in the American guideline, non-vitamin K antagonists anticoagulants, such as new oral anticoagulants (NOAC) dabigatran, rivaroxaban, and apixaban (Class I recommendation, level of evidence B) were added to warfarin (Class I recommendation, level of evidence A) like the preferred therapy. The use of new oral anticoagulants should be considered in patients who cannot maintain a stable INR (Class I recommendation, level of evidence C). The recommendation by the American guideline about warfarin use is based on the results of multiple cohort studies; while the recommendation of using new oral anticoagulants is based in randomized controlled clinical trials comparing to warfarin [32]. However, this aspect is not analyzed and considered by the other two guidelines and must be adequately addressed, given the short range of the INR.
The Canadian guideline prefers new oral anticoagulants rather than warfarin (strong recommendation - High level of evidence), but the American guideline suggests that dabigatran is a useful alternative in some patients over warfarin, while the NICE guideline suggests that the initial dose of dabigatran should be discussed between doctor and patient referring the risks and benefits of this compared with warfarin. It is important to consider that the Canadian guideline got a score of 4.2% in editorial independence, reporting on its website the development of the guidelines through the support of various pharmaceutical industries. This may be related to the fact that since the entry of the new oral anticoagulants in Canada (2008), their use showed an increase in almost double over a period of five years [33].
The developer team of the AHA / ASA 2012 Guideline refused to recommend the new oral anticoagulants over warfarin, citing various reasons such as cost, patient adherence and lack of experience, and specific concerns related to the fact that thrombolysis cannot be given safely to patients taking new oral anticoagulants if their state of anticoagulation is not readily measured [12].
In short, the most controversial issue for the three guidelines was based on three aspects: risk assessment across different risk scores, the use of aspirin, and the inclusion of the new oral anticoagulants in treatment; issues frequently analyzed in several studies [12],[13],[34],[35],[36].
There are marked differences in the guidelines evaluated, showing an association between methodological quality and recommendations in controversy. We cannot say that a guideline is better than another is, but we can say that the NICE guide presents greater rigor in its development including many issues not considered by the other guidelines, among which highlights the patient-centered approach. There is now greater interest from the patients with atrial fibrillation to receive information prepared for them about the treatments received, its relevance, and the prevention of stroke [37]. This which makes NICE guidelines, considered useful in the implementation. This guideline, although has not been updated completely, raises an appropriate approach, and its recommendations are quite permissible.
On the other hand, the American guideline has made a major review and has considered important aspects such as the evaluation of bleeding. The Canadian guideline, despite being updated every two years, does not have adequate methodological rigor, as it is accompanied by recommendations with many doubts, affected by the lack of editorial independence; not considering the requirements to developing clinical practice guidelines based on scientific evidence; unlike the Canadian Hypertension Guideline evaluated in the previous article [11].
Conclusions
In assessing the quality of the atrial fibrillation guidelines, the NICE clinical practice guideline has the best score obtained through applying the AGREE II instrument, followed by the American clinical practice guideline, both of them recommended without changes.
Notes
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Conflicts of interest
The authors completed the conflict of interests declaration form from the ICMJE, and declared not having any conflict of interests with the matter dealt herein. The authors can request the forms from the responsible author or the editorial direction of the Journal.
Funding
The authors declare that no external funding sources.

