Notas metodológicas
← vista completaPublicado el 5 de junio de 2025 | http://doi.org/10.5867/medwave.2025.05.3063
Incorporación de pacientes en el desarrollo de guías de práctica clínica
Incorporating patients in the development of clinical practice guidelines
Abstract
Clinical practice guidelines are a set of recommendations developed systematically and based on the best available evidence. Their purpose is to enable healthcare providers and patients to make the best decisions regarding healthcare interventions associated with a particular clinical condition, considering each patient's specific circumstances. One element that has gained importance recently is considering patient preferences when developing healthcare recommendations to increase adherence to therapeutic measures and patient satisfaction. One way to incorporate these preferences is by including patients or their representatives in developing these tools. Patient participation can take place through their inclusion as members of the development panel and/or at different stages of the guideline development process. This can be achieved through various methods like discussion groups, semi-structured interviews, or surveys. However, challenges still need to be addressed to optimally incorporate patients' perspectives, which, among other reasons, are related to socioeconomic barriers, educational gaps, and the persistence of a paternalistic view of healthcare. This article has been developed in the context of a methodological series on clinical epidemiology, biostatistics, and research methodology carried out by the departments of Research Methodology and Evidence-Based Medicine at the School of Medicine of the University of Valparaíso, Chile.
Main messages
- Including patients and/or their representatives in developing clinical practice guidelines helps ensure that their perspectives are integrated into the recommendations.
- The patient perspective can be incorporated at different stages of clinical practice guideline development, including preparation, development, and/or implementation.
- Some barriers hinder the effective inclusion of patient perspectives, related to socioeconomic and educational factors, and differences of opinion between patients and health professionals.
Introduction
Evidence-based medicine (EBM) has recently marked a milestone in improving clinical decision-making by promoting the best available evidence and improving healthcare quality. In this context, clinical practice guidelines are fundamental in developing healthcare recommendations based on scientific rigor [1].
A critical dimension in the development of clinical practice guidelines is integrating patients' preferences regarding the management of their health. This is important because it can potentially determine the most appropriate recommendations, ensuring that they are relevant, understandable, and respectful of patients' circumstances [2].
Patient preferences can be incorporated in various ways, such as determining them through literature reviews, expert evidence, or involving patient representatives in developing clinical practice guidelines [3].
This review aims to delve deeper into the importance of incorporating patients into clinical practice guidelines, examining the methodologies used for their inclusion and the challenges of this process. The aim is not only to highlight the valuable contribution of patients' perspectives in developing more effective and relevant guidelines, but also to identify the barriers that limit this participation and explore ways to overcome them. The selected sources mainly correspond to consensus statements and recommendations developed by different groups dedicated to developing research frameworks for developing clinical practice guidelines. Reviews on the methodology and results of patient involvement in clinical practice guidelines were also analyzed.
This article is part of a methodological series of narrative reviews focused on essential biostatistics and clinical epidemiology areas. The purpose of this series is twofold. On the one hand, it seeks to synthesize and present relevant research published in leading databases and specialized texts in an accessible manner. On the other hand, it aims to enrich the academic training of undergraduate and graduate students. This effort is led by the Chairs of Health Research Methodology and Evidence-Based Medicine at the School of Medicine of the University of Valparaíso, Chile, underscoring their commitment to quality medical education.
What are clinical practice guidelines?
These instruments are defined as a set of recommendations developed systematically, based on the best available evidence. The objective is to guide healthcare professionals and patients in decision-making regarding which healthcare interventions are most appropriate for addressing a specific clinical condition in specific healthcare circumstances [4].
Who participates in the development of clinical practice guidelines?
For the recommendations provided by clinical practice guidelines to be valid, the development group must be composed of the right people who actively collaborate in analyzing the evidence. These teams usually comprise experts from various clinical and non-clinical disciplines (including statisticians, methodology experts, and computer scientists). However, for some time now, there has been an active push to involve the patients for whom these guidelines are intended in their development, thereby allowing their perspectives to be incorporated [2].
How has the inclusion of patients in developing clinical practice guidelines evolved?
Patient and public involvement in developing clinical practice guidelines has become increasingly important in recent decades. This increase has been driven by the simultaneous development of public participation and evidence-based medicine as fundamental pillars in healthcare policy formulation [5].
A 2020 survey of 52 organizations in 18 countries, with experience developing at least one clinical practice guideline in the previous five years, showed that most included patients, through various methods to incorporate their preferences into the recommendations [6]. Furthermore, a study conducted in Japan showed increased patient participation in developing clinical practice guidelines over time. Of 265 such guidelines analyzed, the percentage involving patients nearly doubled, from 12.5% in the first period (up to 2005) to 32.4% in the fourth period (2016 to 2019). This increase reflects a general trend toward greater patient involvement in decision-making and health policy development [7].
What is the importance of patient participation in the development of clinical guidelines?
Including patients in developing clinical practice guidelines is crucial for many reasons, including ethical considerations. Ensuring patients participate in decisions directly impacting their medical care responds to fundamental principles of equity, autonomy, and justice. It also reinforces the legitimacy of guidelines as people-centered tools. Furthermore, it is considered beneficial for developing these instruments, as it allows for transparency in their creation. In addition, it allows for better identification of the patients' preferences for whom clinical practice guidelines are intended, enabling better determination of the topics that should be focused on and communicating them in a way that patients can understand [2]. Studies have shown that groups that include patient representatives achieve a more patient-centered approach, identifying critical issues and outcomes, and developing questions formulated in accessible terms [8].
Furthermore, active patient participation could positively impact health outcomes by increasing acceptance of and compliance with therapeutic recommendations. This effect can be explained by the fact that patients, by participating in developing guidelines, perceive that their views and preferences have been considered. This reinforces their confidence in the recommendations and willingness to follow them [9].
For all these reasons, in some countries such as the United Kingdom, it is necessary to involve at least two people belonging to the patient group or the public during all stages of the clinical practice guideline development process [10]. The US National Academy suggests the active participation of patients with experience of specific diseases in developing guidelines [10]. Similarly, Canada and Australia require patient and public involvement in approving clinical practice guidelines [10]. For their part, the Spanish Ministry of Health, Social Services and Equality and the Chilean Ministry of Health recommend involving patients or their representatives in developing local clinical practice guidelines [11,12]. Including patients is part of the quality criteria of various standards for developing these tools, such as Appraisal of Guidelines for Research & Evaluation II (AGREE II) and GIN-McMaster Checklist, among others [13,14].
How can patients contribute to the development of clinical practice guidelines?
Developing frameworks for evolving clinical practice guidelines has made it easier to integrate the complexities inherent in the decision-making process. One such framework is GRADE (Grading of Recommendations Assessment, Development and Evaluation), which offers a structured and transparent methodology for developing clinical practice guidelines. This methodology ensures the effective use of evidence and the integration of contextual factors by generating a structured framework for moving from evidence to decision (EtD or Evidence to Decision). This approach improves health decision-making through three main sections: question formulation, assessment of various domains (desirable and undesirable effects, costs, cost-effectiveness, acceptability, feasibility, equity, among others), and conclusions. The certainty and variability of values and patient preferences are among the elements that GRADE considers to determine the direction and strength of the recommendations of a clinical practice guideline. Uncertainty regarding preferences increases the likelihood that the developing panel will determine a recommendation as weak. On the other hand, high variability in preferences decreases the likelihood that a recommendation can be applied uniformly across all patients [15,16].
Another framework proposed for creating clinical practice guidelines is WHO-INTEGRATE, designed to align the process of developing these guidelines with the principles and values of the World Health Organization. This approach introduces elements more explicitly in some aspects, such as the human rights perspective, the social determinants of health, and the sustainable development goals [17].
Despite agreement on its importance and methodologies, there is still no clear and universal consensus on the effective inclusion of patients and the general public in developing clinical practice guidelines. However, one of the most cutting-edge initiatives in this field is provided by the Guidelines International Network (GIN), which offers a practical manual for clinical practice guideline developers (GIN Public Toolkit: Patient and public involvement in guidelines). This tool focuses on collecting international experiences and examples of good practices in public and patient involvement (PPI) to facilitate the effective development of guidelines [18]. For its part, the Spanish Ministry of Health, Social Services, and Equality also has a manual to facilitate the incorporation of patients in developing local clinical practice guidelines at different stages [19]. In addition, the MuSe Consortium, part of Cochrane, is developing a guide that seeks to determine when to involve patients or their representatives in the development of clinical practice guidelines, how to manage potential conflicts of interest between different representatives, and how to evaluate their participation in the development process of these tools [20].
While it is true that there have been significant advances in the development of clinical practice guidelines, specifically in terms of patient involvement [21], it should be noted that a study in Latin America revealed that only a quarter of government-funded clinical practice guidelines in the region included a method for patient participation, with considerable variation between the countries selected [22]. Another Chilean study from 2020 warned about the insufficient inclusion of patients in developing clinical practice guidelines at the local level [23]. This underscores the need for further research and development of approaches that facilitate the effective inclusion of patients, ensuring that their experiences and preferences are adequately integrated into creating clinical guidelines [22].
Who participates as patients in clinical practice guidelines, and how is their participation chosen?
Clinical practice guidelines may include various individuals under the category of patients. One group includes those who have the specific condition addressed by the guideline. However, not all guidelines explicitly detail the stage or severity of these patients' disease. Regarding selection sources, they may be recruited through patient associations, medical registries, or direct contact with the professionals developing the guidelines [24].
In addition, clinical guidelines may consider integrating patient representatives or advocates. These may be members of patient associations, family members, or caregivers who bring valuable perspectives to the guideline development process [24].
How are patients involved in the development of clinical practice guidelines?
There are various methods for integrating patient preferences into clinical practice guidelines. One effective strategy is to directly include patients in working groups, allowing them to contribute actively during the development phase alongside other experts. This approach requires a cohesive group dynamic and that patients are properly prepared with the necessary knowledge and skills (general aspects of the guideline development process, contribution or role expected of them, collaboration dynamics, interpersonal aspects, among others) to ensure a significant impact on the content of clinical practice guidelines [9].
Additionally, primary research can be conducted with patients or their representatives through semi-structured interviews, either individual or group (such as focus groups), patient panels, or online surveys. These strategies are generally used to complement the literature review on patient preferences, especially when local information is lacking. These methods allow patient participation to focus on specific phases of guideline development, highlighting the importance of the team’s mastery of qualitative methodologies. In all the strategies outlined above, training for patients and panelists is crucial to ensure effective and relevant inclusion of patient perspectives [19,25].
Developing clinical practice guidelines can be divided into several stages, facilitating the identification of specific moments when patient participation may be most relevant and beneficial (see Figure 1) [25,26].
Stages of CPG development in which patient preferences or those of their representatives can be included.
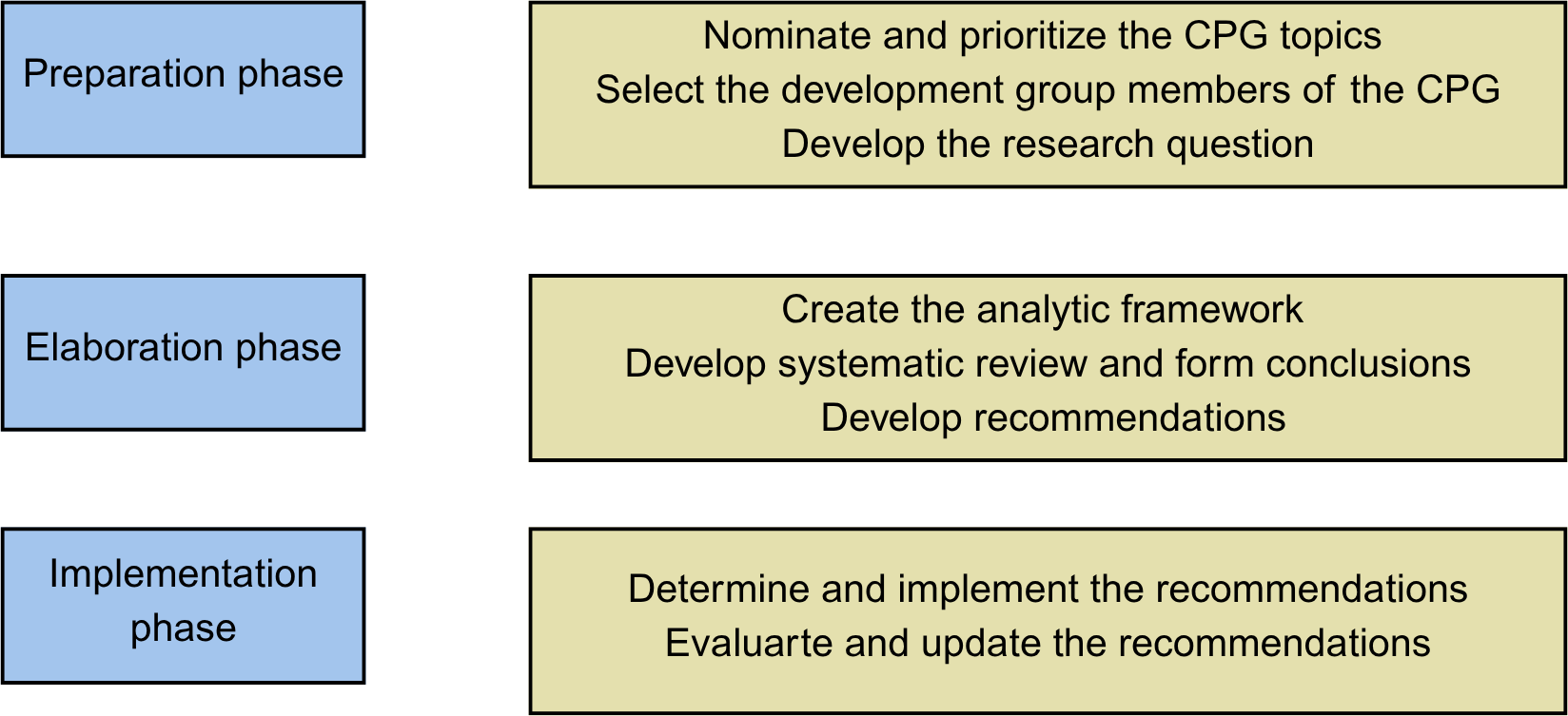
Source: Prepared by the authors.
What are the challenges of including patients in clinical practice guidelines?
Including patients in clinical practice guidelines is a multidimensional challenge, so simply adding them to the team does not always guarantee that their perspectives and needs will be integrated [3]. Several issues act as barriers to their effective inclusion, among which choosing representative patients of the affected population is fundamental. This challenge involves considering variables such as socioeconomic status, cultural diversity, age, and underlying health conditions, ensuring that the voices of all sectors are heard [9].
In addition, differences in educational levels between patients and healthcare professionals can create significant barriers to effective communication. These discrepancies limit patients' ability to fully understand the guidelines' implications and, therefore, to contribute meaningfully. Patients' lack of familiarity with specific knowledge about their disease and difficulty understanding specialized biomedical language exacerbate this situation. This hinders understanding of clinical recommendations and reduces the ability to express their experiences and needs adequately [9,27].
Socioeconomic barriers also play a significant role, as patients from disadvantaged backgrounds may face additional obstacles that limit their participation. These include lack of Internet access, transportation difficulties, and the economic impact of participating in guideline development, including needing caregivers to replace them during their absence or forego other paid work [19].
On the other hand, it is important to consider that patient representative organizations may be subject to conflicts of interest. In fact, many of them receive funding from the pharmaceutical industry. Transparency in this and other types of conflicts of interest is essential for the integrity of the development of clinical practice guidelines [28].
Discrepancies often arise between the perspective of patients and their representatives and the opinion of experts, adding complexity to the process of developing clinical practice guidelines. These differences are exacerbated by the perceptions of some professionals, who consider patients biased or unpredictable, creating a challenging environment for their effective inclusion. Added to this is the unique experience of patients with their disease, which often contrasts with what physicians consider important [27]. In this regard, the role of clinical practice guideline panel chairs becomes essential in facilitating the participation of patients or their representatives with other panel members [18].
Examples of patient involvement in clinical practice guidelines
Example 1
Inclusion in working groups
Depression Following Acute Coronary Syndrome Events: Screening and Treatment Guidelines from the AAFP (American Academy of Family Physicians, USA) [29]
Role of patients:
Panel members who developed the clinical practice guidelines.
Comment:
At least one patient, caregiver, or patient representative was included among the guideline development group members and participated in its development with voting rights on the panel.
Example 2
Inclusion in working groups
Clinical Practice Guideline for the Management of Patients with Gout (Spanish Society of Rheumatology, Spain) [30]
Role of patients:
Members of the clinical practice guideline development panel.
Comment:
Two patients were included in the clinical practice guideline development group from the early stages of the work, alongside healthcare professionals and technicians from the Research Unit of the Spanish Society of Rheumatology.
Example 3
Preparation phase:
Identify the topics to be addressed in the clinical practice guidelines.
Healthcare program to empower patients in returning to normal activities and work after gynecological surgery: intervention mapping as a helpful method for development (VU University Medical Center, Netherlands [31])
Role of patients:
Identifying the needs of the targeted patients.
Comment:
Patients were included in a focus group to identify patient needs, attitudes, and beliefs about postoperative recovery and return to normal activities after gynecological surgery.
Example 4
Development phase:
Recommendations development.
2023 American College of Rheumatology and American Association of Hip and Knee Surgeons Clinical Practice Guideline for the Optimal Timing of Elective Hip or Knee Arthroplasty for Patients with Symptomatic Moderate-to-Severe Osteoarthritis or Advanced Symptomatic Osteonecrosis with Secondary Arthritis for Whom Nonoperative Therapy Is Ineffective (American College of Rheumatology, US) [32]
Role of patients:
Identifying preferred recommendations.
Comment:
A panel of patients who were candidates for or had previously undergone total hip or knee arthroplasty was organized. The patients reviewed the evidence and expressed their preferences, which were then considered by the expert voting panel.
Conclusions
Including patients in developing clinical practice guidelines is becoming imperative to ensure that these guidelines reflect the real needs and preferences of those who will directly benefit from their recommendations. Our review highlights the diversity of methods for integrating patients' views, ranging from their direct participation in working panels to interviews and surveys. However, significant challenges remain in this process, such as the representative selection of patients and adequate training for effective participation.
The patient perspective is crucial throughout the different stages of clinical practice guideline development. Its inclusion not only enriches the quality and relevance of the guidelines but also promotes greater adherence and satisfaction among the users for whom they are intended, along with the ethical importance of inclusion. Some examples of these results can be seen in the inclusion of patients in developing the clinical practice guideline for allergic rhinitis developed by the ARIA (Allergic Rhinitis and its Impact on asthma) initiative to determine relevant outcome indicators for the target population [33]. Added to this is the clinical practice guideline for rheumatoid arthritis from the Japanese College of Rheumatology. It successfully used an online survey to determine patients' preferences regarding their current treatments [34].
However, the lack of an established protocol for patient and public involvement indicates the need for a more structured and systematic framework. Although including patients may increase the cost of producing clinical practice guidelines, which is particularly relevant in lower-income countries, the benefits of this practice in developing better recommendations will likely outweigh these costs.
This discussion highlights the need to overcome methodological and operational challenges to ensure more meaningful patient involvement in developing clinical practice guidelines. Moving in this direction contributes to the design of more people-centered guidelines and reaffirms medicine’s commitment to inclusive, values-based care. Guidelines must authentically reflect the perspectives of those directly affected by their recommendations, such as patients and the general public. This ensures their relevance and usefulness in the real-world context of healthcare.
Finally, we present an infographic (Figure 2) summarizing the key points of this article.
Summary of patient involvement in the development of clinical practice guidelines.


