Review article
← vista completaPublished on July 13, 2023 | http://doi.org/10.5867/medwave.2023.06.2708
Tools of genomic medicine for clinical practice: The example of psychiatry
Herramientas de la medicina genómica para la práctica clínica: el ejemplo de la psiquiatría
Abstract
Most psychiatric disorders are moderate to highly heritable, often with different genetic architectures. Although genetic research in psychiatry has progressed, its findings, interpretation, and impact on clinical psychiatry are unknown to most mental healthcare professionals. This article addresses key genetic concepts to understand some clinical entities, emphasizing genetic terminology and types of mutations. Particularly, we describe the role of heritability in the early days of psychiatry genetic research, the most used study designs, and their main objectives. On the other hand, we review some genetic and genomic databases useful for clinical practice. These include Online Mendelian Inheritance in Man, ClinVar, Ensembl, and The Single Nucleotide Polymorphism Database. Finally, a clinical vignette is presented in which we can apply genomic medicine tools. Since the evidence in psychiatric genetics is based on studies carried out in European or North American ancestral populations, we must develop local studies to increase the knowledge and application of genomic medicine on underrepresented populations.
Main messages
- The genetic basis of psychiatric disorders is significant but not necessarily easy to detect.
- Genomic medicine can be used in psychiatric practice; some applications, such as the family genealogy registry, are simple to do and should be used, while other more specific and complex applications are limited to selected cases.
- Much of the available evidence is based on discoveries made in European populations, which limits its applicability to the Chilean mestizo population. This article provides genetic tools for psychiatric clinical care, including key concepts, useful studies in psychiatry, and genetic databases.
Introduction
Genetics is the scientific study of genes and heritability. Virtually all diseases in humans have some contribution of heritable factors. Particularly for psychiatric illnesses, most of the disorders faced by clinicians have moderate to high heritability [1], with different combinations of causal factors or "genetic architectures" [2]. However, the criteria for establishing diagnoses based on clinical findings follow the descriptive analysis of current psychiatric classifications, such as the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) [3] and the International Classification of Diseases, 11th edition (ICD-11) [4]. These do not directly reflect the disorders' neurobiology, making it difficult to discover the underlying genetic factors.
Psychiatric practice is based on mental examination and clinical records. In this sense, performing an exhaustive anamnesis and a thorough family history is fundamental to identifying the risk of having a psychiatric disorder. Recently, technological tools based on molecular biology and knowledge of the human genome have been incorporated into clinical practice, such as genetic tests, whose validity and clinical applicability have been demonstrated for neurodevelopmental [5] and neurodegenerative disorders [6]. To approach its application and critically evaluate scientific development, some notions about genetic variation and its relationship to interindividual differences in behavior are necessary.
Genetic research, especially genomic research in psychiatry, has increased worldwide. This progress has not been global, as it is mainly based on European populations [7]. The underrepresentation of individuals with other ancestral origins has implications for the applicability of the data [8]. Likewise, this fact influences how mental health professionals inform themselves and how they analyze and intervene in these areas of findings [9]. In the case of Chile, there is a deficit in terms of local genetic information. However, some initiatives seek to contribute to the evidence gap. In particular, the "Chile Genómico" project, carried out by a multidisciplinary scientific team, aims to produce the first genomic systematic characterization of the Chilean population [10].
This review addresses some general concepts of genetics, their applicability in psychiatry, and the use of genetic and genomic databases useful for clinical practice. In addition, a clinical case is presented in this article to help psychiatrists and mental health professionals become familiar with the tools available in genomic medicine. In a future publication, we hope to further explore the applications of these tools.
General concepts in genetics
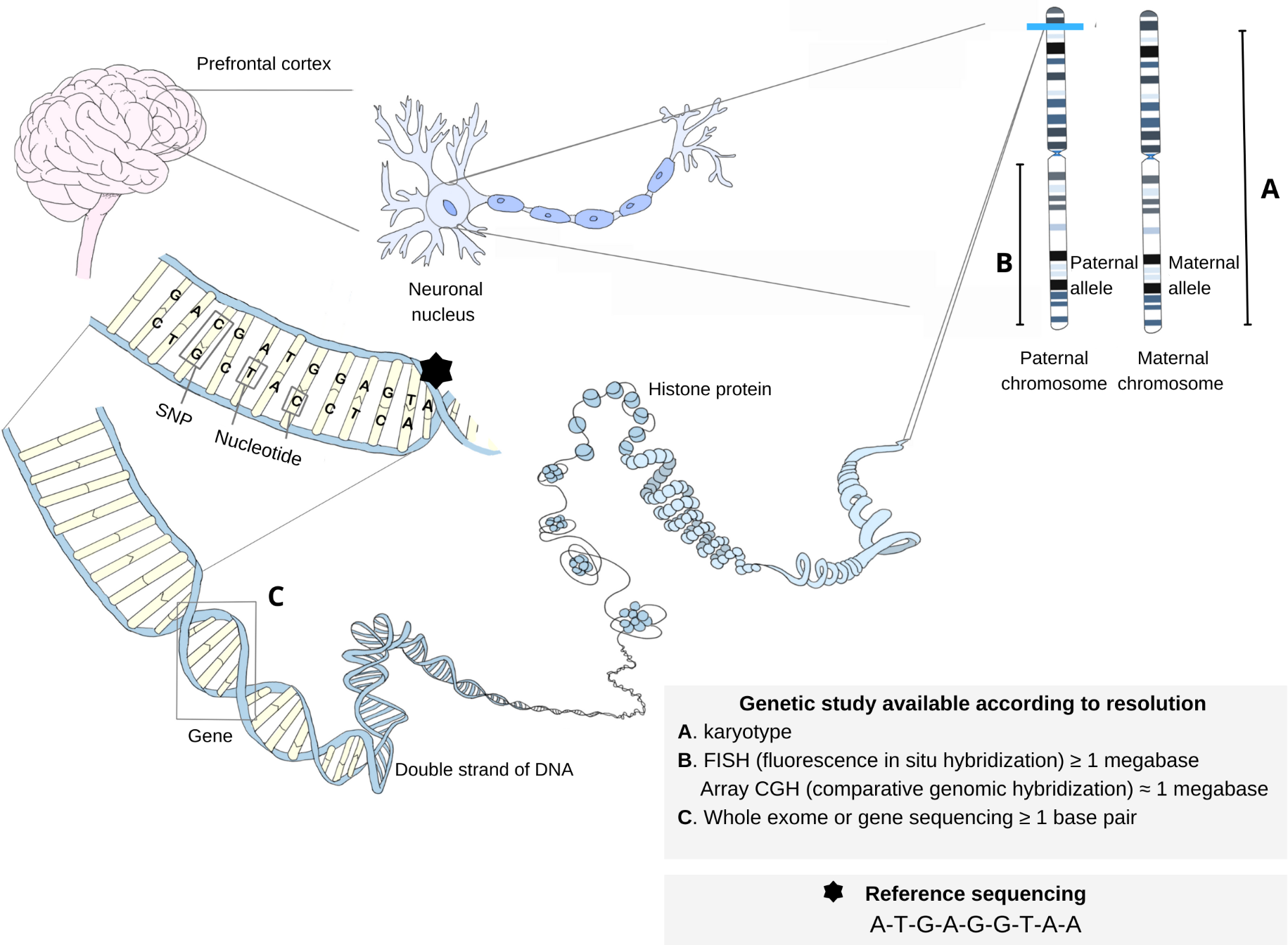
Genetic material is contained in deoxyribonucleic acid (DNA), a molecule composed of nucleotide sequences located in the cell nucleus and mitochondria. During cell proliferation, DNA is replicated to produce identical molecules, then transcribed to synthesize ribonucleic acid (RNA) molecules that will finally be translated in the cytoplasm into amino acid sequences that make up proteins [11]. This sequence of facts was known as the "dogma of molecular biology". However, today it is known that the flow of information does not only follow this direction since there are examples where a DNA molecule can be synthesized from an RNA molecule, revealing the complexity of biological phenomena [12]. DNA molecules are condensed into chromosomes, corresponding to 23 homologous pairs in the human species, 50% provided by the father and 50% by the individual’s mother.
The basic functional unit of heritability is the gene, which corresponds to a DNA sequence responsible for encoding a specific product [13]. It is estimated that in the human species, there are about 20 000 nuclear genes and 37 mitochondrial genes [14,15]. Each human has two copies of each gene on each homologous chromosome. These copies are called alleles and correspond to one maternal and one paternal allele. About 1% of genes encode proteins; the rest are involved in structural and regulatory functions of gene expression [11].
Many genetic concepts are of great relevance to the understanding of clinical entities. Some are described in Box 1 and illustrated in Figure 1.
A variant of a gene inherited from the mother or father. Alleles differ from each other in their nucleotide sequences. The ancestral form of an allele in the population is known as the "wild allele" or "wild type". An example is the serotonin transporter gene (SERT), which has two alleles: the "long" or "L" allele, with 16 tandem repeats of a 23-nucleotide sequence, while the "short" or "S" allele contains 14 repeats and results in lower transcriptional efficiency of the gene. It is associated with multiple negative outcomes, such as suicide risk [16].
Position in which a specific DNA sequence is located on a chromosome. It can range from a single nucleotide to multiple genes. The plural form of locus is "loci" [17]. Example of risk loci are the regions located on chromosomes 3 (3p21.1), 10 (10q21.2), and 15 (15q.14), where single nucleotide polymorphisms were found to increase the risk for bipolar disorder [18].
A physical cluster of genomic variants that tend to be inherited together; thus, a specific haplotype usually reflects a unique combination of variants located proximally on a chromosome [19]. Incidentally, one investigation studied adults with major depressive disorder. The results indicated that carriers of the T-A-T allele haplotype in the gene encoding corticotropin-releasing factor receptor type 1 had worse neurocognitive performance [20].
Genetic variants caused by single nucleotide change at a given locus in relation to a sequence of reference, whose minor allele (the one with the lowest frequency) is observed in at least 1% of the population; if its frequency is less than 1%, they are called single nucleotide variant (SNV). A single nucleotide polymorphism is estimated to occur every 100 base pairs, accounting for more than 10 million in humans. Some of these have more than two known alleles. They may or may not have a significant impact on the phenotype. An example of a single nucleotide polymorphism is rs53576, which is linked to variations in social cognition metrics. This variant is located in an intronic region of the gene encoding the oxytocin receptor (OXTR) [21].
Nucleotide sequence repetitions that include iterations of segments of considerable size (one gene or several genes). It has been proposed that a significant part of the genome could correspond to copy number variants. In schizophrenia, multiple copy number variants have been implicated in its pathophysiology, including 3q29 [22].
Source: Prepared by the authors.
Genetic material structure and studies according to analysis or resolution level.
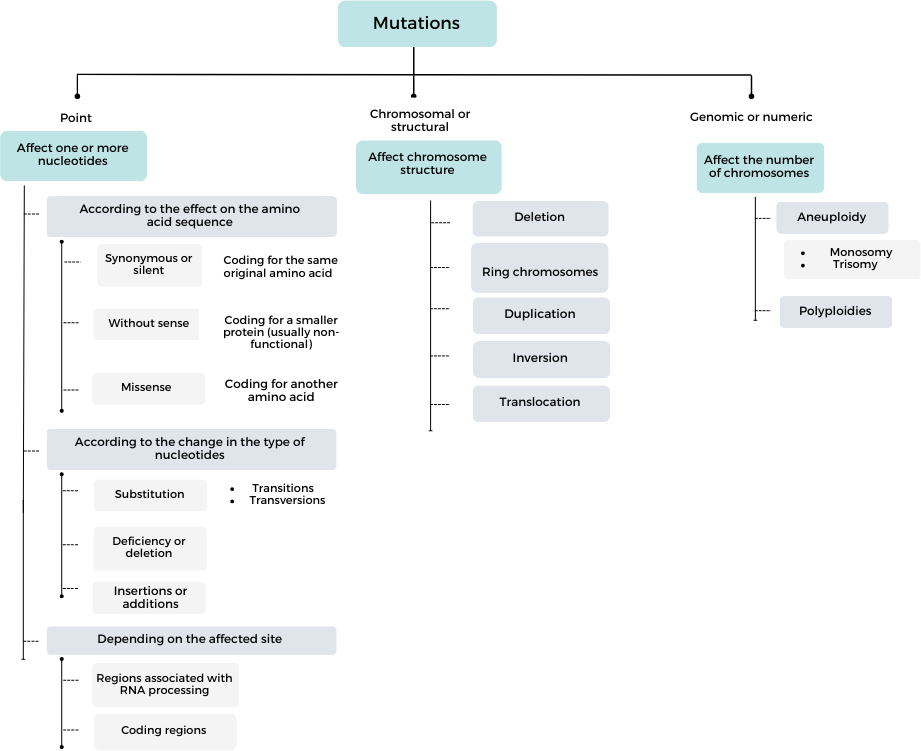
Mutations
Humans share about 99% of their DNA sequence [14]. The different genetic variants in the population arise from mutations, that is, permanent inherited or acquired changes in the DNA sequence. They range from single nucleotide mutations to long nucleotide sequences. Mutations are triggered by errors in DNA replication and repair (spontaneous) or by mutagenic agents, but not all of them have a functional impact [23]; indeed, most mutations have no pathological consequences and can become part of the population gene pool; if the variant has a frequency of more than 1% in the population, it is called a "polymorphism" (Box 1) [24,25]. Different taxonomies of mutations exist in the published literature. Figure 2 shows one of them, which considers the criteria for the size of the observed change and its effect on the product or phenotype.
Genomics and psychiatry
Genomics is a very recent discipline. It is dedicated to studying the complete genome of an organism: its content, structure, and function [11,28]. Genomics combines other disciplines, such as biostatistics and bioinformatics [28]. Specifically, in psychiatry, its application is growing.
The relationship between psychiatric disorders and genetics was raised in the mid-19th century by Morel and Maudsley, who identified hereditary etiology as a substantial contributor to mental illnesses, given the clinical observation of high familial aggregation [29]. However, it was not until the studies of Kallman and Slater in the mid-twentieth century that reliable results were obtained on the importance of heritability in the etiology of severe mental illnesses, such as schizophrenia and bipolar affective disorder [30]. These conclusions were observed more than 50 years ago and have been endorsed by current designs with more data, modern laboratory methods, and statistical analysis. Box 2 lists some of the major research designs in genetics.
Family aggregation studies [31], twin studies [32], and adoption studies [33]. Twin studies, whether monozygotic or identical twins (siblings from the division of a single zygote) or dizygotic twins or twins (siblings from two different zygotes) correspond to a "natural genetic experiment" since they allow differentiating the genetic and environmental contribution to a phenotype by assuming that the siblings have a similar background. These studies estimate concordance, expressed as the proportion of twins sharing a trait in a sample of twins [34].
Segregation studies. This type of analysis is a statistical method to determine a trait’s transmission pattern, either monogenic (Mendelian) or polygenic. Complex segregation analyses enable the study of heritability patterns by incorporating more variables [35].
Linkage studies [36]. This type of study can be used to establish indirect associations. Targeting a candidate gene and identifying polymorphisms in this gene as genetic markers makes it possible to establish a haplotype linked to the pathology. Thus, without functional significance, these genetic polymorphisms would be associated with the disease "indirectly" by being in linkage disequilibrium with the causal variant [34].
Candidate gene association and genome-wide association studies (GWAS) [37–39]. A subtype of association studies that estimate polygenic risk scores. They allow predicting the risk of an individual or population based on the number of risk alleles they carry. They require prior association studies, such as genome-wide association studies, where the risk variants of a given population are identified [40]. Genome-wide association studies are mostly performed in Europe and North America, so the observed risk variants come from a different ancestral origin than the Chilean one. This limits the applicability of score estimation at the local level [41].
Source: Prepared by the authors.
Genetic research in psychiatry groups those studies that seek to demonstrate the existence of heredity in the etiopathogenesis of psychiatric disorders, those that analyze models of heritability, and those that try to establish the mechanisms of genetic changes in symptoms and mental disorders (Box 2) . Evidence shows that the degree of genetic variant contribution is dynamic and considerable for different psychiatric disorders [39]. Thus, for example, some twin studies reveal a heritability of 80% for schizophrenia [42] and bipolar affective disorder [43] and about 50% for major depressive disorder [44].
There are psychiatric disorders that are completely or almost completely determined by a genetic variant, usually rare diseases; on the other hand, other diseases are caused by a combination of many genetic variants, each with a small effect, interacting with each other and with environmental factors. Psychiatric disorders present different combinations of causal factors or genetic "architectures" [45]. Nearly all have moderate to high heritability [2], meaning that the proportion of variation in the population explained by genetic factors exceeds 50% [2]. However, high heritability does not necessarily imply that genetic associations are easy to detect [45].
This background and findings led to the creation of the International Society of Psychiatric Genetics and the Psychiatric Genomics Consortium, which allowed the pooling of genetic data from thousands of people worldwide [46]. In Latin America, genetic research in psychiatry is growing. Despite its population representing 8.4% of the world’s total, only 1.3% of the participants in genome-wide association studies are of Hispanic origin [47]. Nevertheless, some projects and consortia have made progress toward this goal. One of them is the Paisa Project, which has more than 9000 participants from the Paisa population in Colombia [48], characterized by lower genetic variation, given the geographic isolation and demographic expansion of some of its regions [49]. The project aims to evaluate the relationship between the phenotypes of severe mental illnesses and the genetic variants that could contribute to the risk of presenting them. In addition, since 2021, "Misión Origen" [50] has been developing in Colombia. This is a project led by the University of Antioquia and the National Institute of Mental Health of the United States, which aims to become one of the largest genetic studies on severe mental illnesses worldwide, recruiting nearly 100 000 participants, half of them with psychiatric diagnosis and the rest of the participants without a diagnosis. Finally, since 2017 the Latin American Network for the Study of Early Psychosis studies (ANDES), a consortium that includes 15 groups from six countries, including Chile, has been up and running. This network seeks to generate regional genetic research collaborations, allowing knowledge transfer between groups and emphasizing specific aspects of psychosis [51].
Genetic-genomic databases
Genetic databases were created in response to the information overload and the recognition of the scientific knowledge that could be gained. The spread of new techniques for sequencing DNA and proteins, as well as the increasing volume of sequences stored in databases, necessitated the creation of algorithms for cataloging and comparing sequences, from which Margaret Oakley Dayhoff is considered a pioneer since in 1965 she published the "Atlas of Protein Sequence and Structure", which contained the sequence of 65 proteins [52]. In 1980, she created the first computerized database with nucleic acid and protein sequences [53]. In parallel, primary databases such as GenBank were developed in 1982 to store all available DNA sequences [28]. Its growth has been exponential, from 70 thousand base pairs of DNA sequences at the end of 1982 to more than one trillion base pairs in October 2022, doubling every 18 months [54]. It belongs to the National Center for Biotechnology Information (NCBI) of the National Library of Medicine of the United States. It is part of the International Nucleotide Sequence Database collaboration integrated by the DNA Data Bank of Japan and the European Nucleotide Archive [54].
Today, some databases include nucleotide sequences, proteins, genomes, and transcription factors. Some databases that are useful in clinical practice are described below.
Online Mendelian Inheritance in Man (OMIM)
Database on human genes and genetic disorders. It was created in 1960 as a catalog of Mendelian traits and disorders, entitled Mendelian Inheritance in Man (MIM). The online version was created in 1985 and, since 1995, has been available from the National Center for Biotechnology Information (
A tool of interest for clinical practice is the linkage of clinical synopses associated with phenotypes, available in the "gene-phenotype relationship" section. It allows comparison of the clinical features of multiple phenotypes based on the disorders' molecular aspect. This facilitates the elaboration of prognoses and/or particular treatments for patients.
ClinVar
It was created in 2012 by the National Center for Biotechnology Information to obtain clinically relevant interpretations of human genetic variations [56]. ClinVar includes mostly single nucleotide variants, short insertions or deletions, and more than 16 000 structural type variations [57]. The ClinVar search can be performed in the National Center for Biotechnology Information database suite (
ClinVar content includes details of the variant, submitter (who enters the record into ClinVar), gene(s) affected by the variant, associated disease or condition and its interpretation, review status, and evidence. In addition, there are links to other databases such as ClinGen, GeneReviews, Genetic Testing Registry, MedGen, and Online Mendelian Inheritance in Man and Variation.
Ensembl
This genome browser was developed in 1999 by the European Bioinformatics Institute (EMBL-EBI) and the Wellcome Trust Sanger Institute [58] to provide automatic genome annotation and integrate these annotations with other available biological data. It is useful if information is needed on variations in the sequence of a gene associated with a disease or condition, search for homologous genes in other species, explore the region around a gene of interest, and search for sequences associated with gene regulation, among others. Since 2009, the "Ensembl Genomes" project arose to provide taxonomic reference data and thus an evolutionary context for a better understanding of genes. It includes genomes of plants, fungi, metazoans, bacteria, and protists.
Access is from their website (
Ensembl features several tools of interest, such as the BLAT/BLAST- search engine, which allows searching by gene or protein sequence; the variant effect predictor, which analyzes variants and can predict their functional consequences; and Biomart, which allows exporting customized datasets.
The Single Nucleotide Polymorphism Database (dbSNP)
It is a database of single nucleotide polymorphisms created by the National Center for Biotechnology Information in collaboration with the National Human Genome Research Institute in 1999 [59]. It includes other variations, such as small insertions/deletions, microsatellites, or short tandem repeats [60].
The Single Nucleotide Polymorphism Database can be searched directly from their website (
Clinical vignette
We now outline a clinical vignette based on the fictitious case of a 13-year-old patient consulting for mental symptoms (Box 3). For his management, it was decided to perform genetic studies.
A 13-year-old male patient with a history of low IQ according to a previous psychometric test and attention deficit disorder. He has a family history of a father with bipolar affective disorder, diagnosed at 25 years of age. The mother mentions that three months ago, she noticed a noticeable change. Her son has lost interest in going to school, hanging out with his friends, and doing things he used to enjoy. She comments that he used to enjoy playing soccer but no longer shows excitement for his matches. She indicates that he seems isolated and more serious, so she fears that he may be going through depression. She is concerned that she is not the only one who notices this, but her son’s teacher commented that he was suffering bullying at school. Despite this, he does not seem to be affected by these attitudes. In addition, during the last month, the mother noticed "strange behaviors", such as talking to himself or worrying excessively about his three-year-old cat, not wanting to be separated from her to the point of asking to take her to school every day for fear that something might happen to her when she is alone. The patient indicates that he is sure that his cat is on the verge of death because she sleeps more than usual, so he does not want to waste a single minute with her. He also says the veterinarian hates him since he does not want to tell them "the truth" about his cat, insisting she is healthy. The poverty of ideas in his speech is striking. The physician rules out a depressive episode, as he does not show sadness, irritability, behavioral alterations, sleep, appetite, or other suggestive aspects. No restricted interests or previous socialization problems were observed. A psychotic episode is suspected, probably from the schizophrenia spectrum, due to the associated negative symptomatology (affective flattening, social withdrawal, anhedonia, alogia). Given the presentation characteristics, earlier than the usual age of onset, and characterized by concomitant executive failures, in addition to the history of a first-degree relative affected by a similar pathology, the treating physician suspects a monogenic etiology.
After a search in Online Mendelian Inheritance in Man, he identifies chapter #181500, which refers to "Schizophrenia, with or without a mood disorder". In the section on "Cytogenetics," the most frequent mutations involved in cases of schizophrenia that share presenting features with the case of interest are indicated, which correspond to microdeletions or microduplications that may involve one of several chromosomal regions. Since there are no clinical records to suspect a specific chromosomal region, an array comparative genomic hybridization (array CGH) is requested to look for chromosomal rearrangements with high resolution and genome-wide (Figure 1). However, this study was negative, ruling out the presence of microdeletions or chromosomal microduplications. For this reason, it was decided to perform an exome sequencing study to identify changes in one or more nucleotides undetected by comparative genomic hybridization. The exome sequencing study identified a single nucleotide variant in the SETD1A gene. In a subsequent article, we will discuss the clinical interpretation of this variant.
This vignette shows how progress can be made in understanding the etiology of a mental disorder using some of the available tools.
Source: Prepared by the authors.
Conclusions
This review addresses concepts, findings, and some useful tools to apply genetic knowledge in psychiatry. Although its development has progressed slowly, it is positioned as an area of relevance given its findings in the understanding and possible management and prevention of psychiatric disorders.
Extensive evidence exists on the genetic basis of psychiatric disorders, and sophisticated technologies for accessing large amounts of genetic information on individuals at costs that make it feasible to compare tens of thousands of individuals. However, the most basic tool of genomic medicine, which has universal application, is family history, an element clinicians should always consider. Selected cases, defined as atypical presentations by criteria such as onset age, clinical manifestations, or presence of family history, will require the performance of specific studies. We will address the review of these studies in a complementary article to this one.
The present article recapitulated the concepts essential for understanding how genetic variation can affect the risk of presenting a psychiatric disease.
We hope to contribute to understanding the relevance of conducting genetic research in Chile, which is crucial to avoid extrapolating results in populations with different ancestral origins. With this, we aim to provide access to precision medicine for people with psychiatric disorders.

