Artículo de revisión
← vista completaPublicado el 8 de abril de 2025 | http://doi.org/10.5867/medwave.2025.03.2938
Colonización oral y orofaríngea por Enterobacteriaceae: revisión narrativa sobre su epidemiología e impacto clínico
Oral and oropharyngeal colonization by Enterobacteriaceae: A narrative review on its epidemiology and clinical impact
Abstract
The family encompasses a large group of Gram-negative bacteria of the order Enterobacterales, which are habitual residents of the intestinal and urinary tracts, behaving only as allochthons in the oral cavity. There is no consensus regarding the prevalence, determinants and consequences of high aero-digestive colonization by this family of bacteria. In light of this, a narrative review was conducted on the epidemiology and clinical impact of oral and oropharyngeal colonization by , differentiating by age groups. A bibliographic search was conducted in four digital databases: PubMed/MEDLINE, Scielo, Google Scholar and Cochrane library of clinical and preclinical studies published in the last twenty years (2003 to 2023), on asymptomatic colonization of in the upper aero-digestive tract, its determinants and clinical impact. Oral and oropharyngeal colonization by was 38.24 and 39% on average, respectively. The genera spp. (68.75%), spp. (68.75%), spp. (43.75%), spp. (25%), and spp. (25%) were the most prevalent taxonomic groups in the oral cavity, especially in children and adolescents, while spp. (22.5%), spp. (15.5%) and spp. (8%) were the most prevalent in the oropharyngeal area of senile subjects. This colonization is shown to be associated with an increased risk of infectious and inflammatory episodes such as pneumonia, inflammatory bowel disease, periodontal disease, and progression of renal failure; with determinants that differ depending on age, with periodontal disease being a shared risk factor for senile and non-senile groups. The presence of enterobacteria in the upper aerodigestive tract is significant, predominating in children and adolescents, promoted by various factors that differ according to age and with systemic consequences of an infectious or inflammatory nature in certain types of hosts. Its role in the pathogenesis of oral diseases such as periodontitis is still not possible to specify.
Main messages
- Currently, there is no existing literature that reviews the evidence on the determinants and both local and systemic clinical impacts of asymptomatic oral and oropharyngeal Enterobacteriaceae colonization.
- This study aims to summarize and analyze the available evidence regarding the consequences, prevalence, and determinants of colonization by this group of Gram-negative bacteria in the oral cavity and oropharynx—uncommon environments for these microorganisms—while differentiating between elderly and non-elderly populations.
Introduction
The Enterobacteriaceae family comprises a large group of Gram-negative bacteria of the order Enterobacterales, consisting of more than 30 genera and over 100 species, with a significant impact on medical, veterinary, and public health. In humans, they behave as habitual residents of the intestinal and urinary tracts, associated with a wide range of clinical syndromes, including foodborne enteritis and zoonotic infections [1]. According to the Centers for Disease Control and Prevention (CDC), members of the Enterobacteriaceae family are listed as an important pathogen for three of the four main categories of infections linked to healthcare, causing between 23% and 31% of these infections in adult, pediatric, and oncology wards [2,3]. In addition, according to the World Health Organization (WHO), Enterobacteriaceae resistant to carbapenems and third-generation cephalosporins are in third place among the ten priority pathogens. New antibiotics are needed to combat the extensive resistance of these bacteria [4].
Enterobacteria behave as non-natives in the oral cavity. Their presence becomes significant and persistent in local inflammatory conditions that promote their clonal expansion [5,6], or in systemic conditions that affect oral ecology, such as immunosuppression associated with aging [7]. It can also become significant in adolescents with recurrent respiratory symptoms [8]. It has been reported that a microbiome with an overabundance of enterobacteria and their products can generate chronic inflammation, accompanied by increased epithelial permeability. This favors the translocation of these bacteria and their metabolites to other organs via the hematogenous and enteric routes. This condition has been linked to an increased risk of non-communicable diseases such as chronic kidney disease [9], diabetes mellitus [10], fatty liver [11], respiratory [12], cardiac [13], neuronal [14] and inflammatory bowel disease [15], obesity [16] and cancer [17]. This association is based on the theory of germ organs [18].
Based on studies from Brazil, the United States, the United Kingdom, Germany, Australia, India and Malaysia, the oral prevalence of Enterobacteriaceae varies widely according to geographical region, age group, racial status, use of prostheses, parafunctional habits, medical status, periodontal status and methodologies used, ranging from 0 to 72% [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. This group of facultative anaerobic, Gram-negative, bacillary bacteria is among the most frequently found in the oral cavity, particularly amongst Asian individuals over the age of 50, people with dental prostheses, and those with comorbidities such as chronic kidney disease. The species Raoultella ornithinolytica, Enterobacter cloacae, Klebsiella pneumoniae subsp. pneumoniae, and Klebsiella oxytoca are the most commonly isolated [26,27]. In fact, it has been demonstrated that oral microbiota under dysbiosis contains a higher relative abundance of Enterobacteriaceae compared to other mucosal sites [33]. This work aims to provide an updated review summarizing all published evidence over the past twenty years regarding asymptomatic Enterobacteriaceae colonization in the upper aero-digestive tract, along with its determinants and clinical impact, differentiating between elderly (over 65 years) and non-elderly (under 65 years) populations. This review is based on the aforementioned background, despite the lack of literature reviews summarizing available evidence on the determinants and local and systemic clinical impact of asymptomatic oral and oropharyngeal Enterobacteriaceae colonization
Methods
A comprehensive review was carried out of the relevant scientific literature published in the last twenty years (from 2003 to 2023), in English and Spanish, and full text, on the epidemiology and clinical impact of oral and oropharyngeal colonization by gram-negative bacilli belonging to the Enterobacteriaceae family. For this purpose, we conducted a search across four digital databases: PubMed/Medline, SciELO, Google Scholar, and the Cochrane Library. The following MeSH terms and search combinations were used: Enterobacteriaceae AND oral colonization OR oropharyngeal colonization AND risk factors AND clinical importance OR medical importance.
The inclusion criteria for this review were:
-
Preclinical studies in animal models.
-
Observational, descriptive, or analytical clinical studies.
-
Systematic reviews with or without meta-analysis of descriptive cross-sectional or analytical case-control and cohort studies.
The exclusion criteria were:
-
Experimental clinical studies.
-
Letters to the editor.
-
Narrative reviews.
-
Systematic reviews with or without meta-analysis of clinical trials.
Microsoft Excel 2019 was used to organize and process the selected evidence, which was analyzed using the deductive reasoning model. Descriptive statistics were applied to summarize the frequency data and reach a consensus regarding the oral and oropharyngeal colonization rates reported in the different primary studies collected.
Results
Based on the inclusion criteria used, 18 studies were selected to meet the objective of the present review. Of these, 15 were clinical studies (six with a case-control design and nine descriptive cross-sectional studies), and 3 were preclinical studies, carried out in animal models.
Regarding the clinical studies, 13 of them studied the oral colonization rate of Enterobacteriaceae. Only two studies evaluated the presence of this group of bacteria in the oropharynx; a total of 18 794 subjects participated in these studies, with 65.04% female and 34.96% male participation, aged between 6 and 101 years. Seven of these studies used saliva to search for enterobacteria in the oral cavity, five used biofilm samples recovered from periodontal pockets or the back of the tongue, and one study used biofilm accumulated on retainers. Only two of the selected clinical studies used oropharyngeal swabs to investigate the presence of this group of bacteria. Regarding the method of microbial identification, 12 of the 15 clinical studies selected used phenotypic identification systems (Table 1), two used mass spectrometry (Matrix-Assisted Laser Desorption/Ionization-Time-Of-Flight/ MALDI-TOF) combined or not with phenotypic methods such as Vitek 2 and molecular techniques such as 16S ribosomal DNA sequencing (Table 1), and one study used only a molecular method based on DNA hybridization.
Epidemiology
Oral colonization of Enterobacteriaceae: the rate of Enterobacteriaceae oral colonization, independently of exposure factors and demographic variables, was 38.24% on average (Table 1). Additionally, the review of selected articles allowed us to establish the prominence of subgingival niches as a reservoir of opportunistic pathogens, including enterobacteria. In fact, three Latin American studies and one European study report an average colonization rate of 29.25% of Enterobacteriaceae in subgingival biofilms in systemically healthy subjects with varying degrees of periodontal disease [30,40,41,42].
Considering the age of the participating subjects, only ten studies specified this information. In five of them, the samples were obtained from children and adolescents, whose ages ranged from 6 to 17 years. Four studies evaluated the oral colonization of enterobacteria in young and middle-aged adults, while only one study examined colonization across a wide age range, from children to older adults (Table 1). The prevalence of Enterobacteriaceae colonization in the group of children and adolescents was 53.72%, compared to 39.13% for adults and middle-aged people.
From a taxonomic point of view, the bacterial genera identified in all the studies included in the analysis were Enterobacter spp., Klebsiella spp., Raoultella spp., Citrobacter spp., Serratia spp., Escherichia spp., Pantoea spp., Proteus spp., Rahnella spp., Shigella spp., and Yersinia spp. Of these, the genus Enterobacter spp. (68.75%), Klebsiella spp. (68.75%), Escherichia spp. (43.75%), Citrobacter spp. (25%), and Pantoea spp. (25%) were the most prevalent taxonomic groups in the oral cavity of the sample. In addition, the genera Enterobacter spp., Klebsiella spp., Citrobacter spp., and Pantoea spp. had the highest representation of species colonizing the oral cavity (Figure 1). The species Enterobacter cloacae was identified in 75% of the evaluated studies. In contrast, the species Klebsiella pneumoniae, Escherichia coli, and Enterobacter aerogenes showed the exact prevalence, appearing in 40% of the assessed studies. This was followed by Enterobacter gergoviae, which was reported in 30% of the analyzed studies. When considering the taxonomy of Enterobacteriaceae within the subgingival biofilm, the studies analyzed reveal that the genera Enterobacter, Serratia, and Klebsiella are the dominant groups in the periodontal sulcus and pocket. At the species level, Enterobacter cloacae, Serratia marcescens, and Klebsiella oxytoca exhibit the highest prevalence rates at periodontal sites, both in healthy and sick individuals [30,40,41,42].
Prevalence, determinants, and effects of asymptomatic colonization of
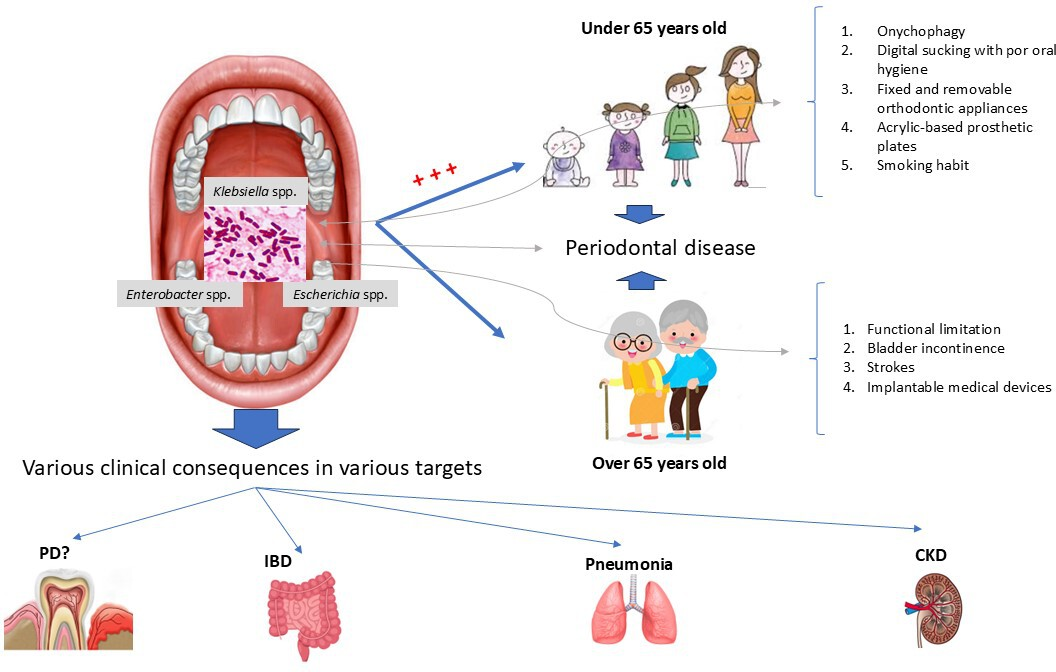
Note: based on the review carried out, the genera
Source: Prepared by the authors with Microsoft Power Point 2019.
Regarding the phenotype, only 7 of the 10 studies selected for this section evaluated antimicrobial activity. In this regard, the least active drugs against this group of bacteria were amoxicillin (85% of isolates resistant), followed by ampicillin (71% of isolates resistant), amoxicillin plus clavulanic acid (42.8% of resistant isolates), ciprofloxacin (35% of resistant isolates), and trimethoprim sulfamethoxazole (24% of resistant isolates).
When analyzing the research that studied antimicrobial susceptibility in enterobacterial isolates derived exclusively from subgingival niches, it was found that the species Serratia spp., Enterobacter spp., and Klebsiella spp., frequently exhibit intrinsic resistance to routinely administered beta-lactams such as ampicillin, amoxicillin, amoxicillin-clavulanic acid, and first- and second-generation cephalosporins [30,40,41].
Oropharyngeal colonization of Enterobacteriaceae: the rate of Enterobacteriaceae colonization in the oropharynx, regardless of exposure factors and demographic variables, was 39% on average (Table 1). Considering the age of the participating subjects, only two studies provided this information. In these studies, the samples were obtained from elderly people, whose ages ranged from 55 to 101 in the first study. In the second study, all participants were over 65 years old. The prevalence of oropharyngeal colonization by Enterobacteriaceae in the first group was 38% compared to 40% for the second group (over 65 years old, Table 1). From a taxonomic perspective, the bacterial genera identified in all studies and included in the analysis were Enterobacter spp., Klebsiella spp., Escherichia spp., Raoultella spp., Proteus spp., and Rahnella spp. Of these, the genera Klebsiella spp. (22.5%), Escherichia spp. (15.5%) and Enterobacter spp. (8%) were the most prevalent taxonomic groups in the oropharyngeal area of all subjects evaluated. Additionally, these genera had the highest representation of species in this ecological niche (Table 1). The species Klebsiella pneumoniae, Escherichia coli, and Enterobacter aerogenes were identified in 100% of the included studies, whereas Proteus mirabilis, Raoultella planticola, and Rahnella spp. were identified in only 50% of the evaluated studies.
Regarding the phenotype, only one of the two studies selected for this section evaluated antimicrobial activity. In this regard, only Escherichia coli exhibited a multidrug-resistant phenotype, with ampicillin, piperacillin, cefazolin, ceftazidime, cefotaxime, cefozopran, cefpodoxime, and ciprofloxacin being the least active drugs against isolates of this species. Strains of Klebsiella pneumoniae showed resistance only to ampicillin; Serratia marcescens was resistant to ampicillin and cefazolin, while Enterobacter cloacae showed resistance to ampicillin, cefazolin, ceftazidime, cefotaxime, cefpodoxime, and fosfomycin. The least active antibiotics against Klebsiella aerogenes were ampicillin, cefazolin, ceftazidime, and cefotaxime. Finally, Morganella morganii showed resistance to ampicillin, cefazolin, cefpodoxime, and fosfomycin. Only isolates of Rahnella spp. and Raoultella planticola showed sensitivity to all the antibiotics tested [36]. On the other hand, in the referenced study, the antibiotics that demonstrated high performance against isolates of enterobacteria derived from the oropharynx were carbapenems, piperacillin-tazobactam, ampicillin-sulbactam, amikacin, and sulfamethoxazole [36].
Colonization determinants
The analysis revealed determinants associated with the asymptomatic colonization of this family of bacteria in the mouth and oropharynx, taking into account the patients' ages. In subjects over 65 years of age, the following factors were identified: functional limitations due to neoplastic, respiratory, and cardiac diseases; deterioration or terminal clinical status; bladder incontinence; cerebrovascular accidents (stroke or cerebral hemorrhage); and the presence of percutaneous endoscopic gastrostomy tubes [35,36] (Figure 1). In individuals under 65 years of age, the determinants recognized for oral and oropharyngeal colonization included onychophagia, the use of orthodontic appliances (fixed and removable), acrylic-based prosthetic plates, digital suction, a high dental plaque index, and smoking. Periodontal pockets were identified as a determinant in both age groups [27,28,30,32,38,39,42] (Figure 1). Regarding this last determinant, the evidence collected from 2007 to 2022 agrees on the medical relevance of subgingival biofilm as a reservoir for opportunistic pathogens, including Pseudomonas aeruginosa, Acinetobacter spp., and other enterobacteria [40,42].
Excluding the age factor, severe chronic kidney disease associated with peritoneal dialysis has also been recognized as a promoter for the oral and oropharyngeal colonization and proliferation of enterobacteria. This is supported by the accumulation of uremic toxins, which alter the oral environment and, in turn, exert selective pressure on the oral microbiome. All this leads to dysbiosis, with a significantly higher prevalence of urease-producing enterobacteria. This oral dysbiosis can potentially lead to an increase in resistant infectious and inflammatory episodes, which can contribute to the progression of kidney disease and its mortality rate [26,34].
Medical-dental importance
Based on the reviewed evidence, the medical-dental risks recognized as being linked to asymptomatic colonization of enterobacteria were:
Pneumonia in patients on mechanical ventilation or with bronchoaspiration: The presence of antimicrobial-resistant bacteria in the oropharyngeal area poses a potential risk of pulmonary infections, such as aspiration pneumonia, in residents of long-term care facilities [34]. Additionally, evidence shows that oral colonization by Klebsiella pneumoniae is associated with an increased risk of aspiration pneumonia in susceptible individuals [38] (Figure 1).
Inflammatory bowel disease: Certain species of Enterobacteriaceae, such as Klebsiella pneumoniae and Escherichia coli, have been linked to the pathogenesis of inflammatory bowel disease [43]. Similarly, in 2017 preclinical research led by Honda Kenya showed that the intestinal translocation of oral strains of Klebsiella spp., (from the saliva of healthy subjects and subjects with inflammatory bowel disease/Crohn’s disease and ulcerative colitis) in gnotobiotic animals, produced an immune differentiation polarized to a Th1 phenotype in the colonic lamina propria of the mice. Additionally, the researchers determined that the oral Klebsiella strains were resistant to multiple antibiotics and could only mediate efficient colonization in animals with intestinal dysbiosis, generated by previous antibiotic treatment. However, this immune polarization to Th1 phenotype only translated into an inflammatory response in Il10-/- mice, or in mice genetically prone to colitis [37] (Figure 1).
The search structure used in this review allowed us to obtain a second preclinical piece of evidence demonstrating the capacity of Enterobacteriaceae isolates (Escherichia coli, Salmonella Enteritidis LB, and Salmonella Typhimurium) to generate early gastrointestinal inflammation after oral inoculation into birds on the day of hatching [44].
The third preclinical evidence obtained and analyzed in this review demonstrated, using a murine model of ligature-induced periodontitis, the expansion of oral pathobionts belonging to the Enterobacteriaceae family. This situation promoted intestinal inflammation in mice with dextran-sodium sulfate (DSS)-induced colitis. Specifically, the study demonstrated that oral inflammation facilitated the proliferation of Enterobacteriaceae, including Klebsiella spp. and Enterobacter spp., which subsequently colonized the intestines of genetically susceptible mice (interleukin-10-/-), thereby exacerbating pre-existing intestinal inflammation. However, such colonization did not occur in wild-type or immunocompetent B6 mice. Additionally, the study showed that direct intestinal colonization by these oral pathobionts induced a strong production of interleukin-1B via inflammasome activation in macrophages of the inflamed intestine, further aggravating the intestinal pathology. Nonetheless, overcolonization by these oral pathobionts was not observed in mice with healthy intestines, even in the presence of periodontitis. Based on these findings, the researchers suggested that at least two microbial events are required for oral pathobiont-driven intestinal inflammation to occur:
a. Oral dysbiosis is the first prerequisite, a condition that increases the number of pathobionts in the oral cavity.
b. Intestinal dysbiosis with decreased resistance to colonization by oral pathobionts, which can pass through the gastric barrier into the intestinal habitat.
It is worth noting that the Klebsiella strains obtained by the researchers in this study, from mice with periodontitis, correspond to antibiotic-resistant strains. In this regard, the authors suggested that the potential risk of using antibiotics to mediate intestinal inflammation may promote the ectopic colonization of oral pathobionts in the dysbiotic intestinal environment [45].
Worsening of the systemic condition of patients with chronic kidney disease:
Based on the results obtained by Costa et al. [26], in a recently published case-control observational study, the higher prevalence and diversity of clinically relevant Enterobacteriaceae species in the oral cavity of patients with chronic kidney disease undergoing peritoneal dialysis represents a greater risk of infections and inflammatory episodes, contributing to the progression of renal disease and its mortality rate. This is due to significantly higher colonization levels of the species Raoultella ornithinolytica in the saliva of renal patients compared to controls, which is favored by the significant increase in saliva pH, urea, and ammonium in the case group. Furthermore, the study demonstrated the existence of multidrug resistance, exclusively in isolates from patients with renal disease. This implies a higher risk of severe morbid infections in this patient group, contributing to an increase in the mortality rate (Figure 1).
A possible pathogenic role in periodontal disease:
A relevant study published in 2016 by Vieira Colombo et al. [42] determined the prevalence and levels of medically relevant pathogenic species in the subgingival microbiota of individuals with varying periodontal statuses. This demonstrated that the bacterial species most related to periodontal inflammation and tissue destruction, both at the site and patient level, were Neisseria spp., Pseudomonas aeruginosa, Olsenella uli, Hafnia alvei, Filifactor alocis, and enterobacteria such as Serratia marcescens. In addition to this evidence, Pontes et al. [40], who studied the prevalence and antimicrobial susceptibility of Gram-negative bacilli in pathological periodontal sites, were able to conclude, based on their observations, a high prevalence and diversity of gram-negative bacilli with low sensitivity to beta-lactams, integrating the subgingival microbiota associated with periodontitis (Figure 1).
Discussion
Enterobacteriaceae is a family that encompasses several bacterial species of clinical relevance, as many of them exhibit high levels of antimicrobial resistance, mediated by enzymatic and non-enzymatic mechanisms. These mechanisms are encoded in intrinsic genes or are acquired by mobile genetic elements [46]. The WHO classifies Enterobacteriaceae resistant to third-generation cephalosporins and carbapenems as priority pathogens [47]. The mechanism of antibiotic resistance with the greatest clinical and therapeutic impact is, for many gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomona aeruginosa, Proteus mirabilis, Enterobacter cloacae and Aeromonas spp, the production of β-lactamase enzymes (extended-spectrum β-lactamases and carbapenemases) that hydrolyze the β-lactam ring of a wide group of antimicrobials, from penicillins, first, second and third generation cephalosporins, to monobactams and carbapenems. Depending on the type of expressed enzyme, this resistance can extend to suicide inhibitors such as clavulanic acid, sulbactam and tazobactam, and can even include other classes of antibiotics such as trimethoprim-sulfamethoxazole, quinolones and aminoglycosides [46,48].
From a morphological, structural, and physiological perspective, Enterobacteriaceae correspond to Gram-negative, facultative anaerobic, non-spore-forming bacilli. They are native to the human and animal intestinal tract. Infections caused by these bacteria frequently result from translocation from a habitual niche to an unusual one [19]. In addition, diseases caused by strains that produce extended-spectrum β-lactamases or carbapenemases are associated with increased mortality rates, prolonged time to effective therapy, extended hospital stays, and high healthcare costs [49,50]. In parallel, it has been established that oral microbiota under dysbiosis contains the highest relative abundance of Enterobacteriaceae compared to other mucosal sites [51]. In light of this data, we consider it relevant to summarize and analyze the available evidence on the consequences, prevalence, and determinants of colonization by this group of Gram-negative bacteria in an unusual niche for them, such as the mouth and oropharynx, distinguishing between senile and non-senile groups. The aim of this is to recognize risk groups for carriage, and potentially for the development of opportunistic infections mediated by strains carrying antibiotic resistance genes.
Based on the review, no significant differences were found in the rate of Enterobacteriaceae colonization between the oropharynx and the oral cavity, with an average of around 40% in both cases, regardless of demographic and clinical variables. This result is comparable to the statistics reported by Philpot et al. [25], a study conducted in Malaysia and published in the 1980s. In the same study, oral carriage of Enterobacteriaceae was reported at 36% for adults and 46% for children. However, studies dating from the 1970s [21] and 1990s [52] reported oral colonization rates for enterobacteria of less than 20%. These data suggest an increase in the prevalence of oral carriage of Enterobacteriaceae over the last twenty years.
When comparing the oral epidemiology of Enterobacteriaceae with that reported in other sites, such as rectal mucosa, a quantitative difference is observed, but not a qualitative one. In fact, the study by Padilla et al. [53] reported a prevalence of 14.4% rectal colonization with enterobacteria, primarily among subjects with a history of surgical intervention or those who had previously received antibiotics. Regarding the species profile, the study by Padilla et al. reported a high rate of isolation of Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli. These results align with our review, indicating that the most frequent taxa in both oral and oropharyngeal colonization were Enterobacter spp., Klebsiella spp., and Escherichia coli. This phenomenon highlights the significant role of fecal-oral transmission in the oral-rectal colonization of Enterobacteriaceae.
When considering the influence of demographic variables on the rate of oral enterobacterial colonization, it was found that children and adolescents are more likely to be colonized than young and middle-aged adults. This tendency is likely due to the higher prevalence of parafunctional habits, such as onychophagia, in children and adolescents, as well as poor hygiene habits [27]. There are no studies comparing enterobacterial colonization in the oropharynx among different age groups. The available evidence shows that between 38 and 40% of the elderly and senescent adult population are colonized by enterobacteria in this location, contrasting with the frequency of colonization reported in other sites. Indeed, a rectal colonization rate of 14.4% has been reported in the elderly population [53]. This difference in enterobacterial load between the rectum and oropharynx in a vulnerable age group, linked to immunosenescence, explains the high frequency of pneumonia reported in elderly hospitalized patients [54]. In this population group, it is recognized as a significant risk factor for oropharyngeal colonization with antibiotic-resistant bacteria, such as β-lactamase-extended-spectrum Enterobacteriaceae, and a personal history of cerebrovascular events. On the other hand, several studies demonstrate the efficacy of professional oral care in reducing morbidity and mortality associated with nursing home-acquired pneumonia [54,55].
At the taxonomic level, the bacterial Enterobacteriaceae family that showed dominance in both the oral cavity and the oropharynx was the genera Enterobacter spp., Klebsiella spp., and Escherichia spp. However, at the species level, slight discrepancies were observed between the two habitats, with Enterobacter cloacae being the most prevalent species in the oral cavity and Klebsiella pneumoniae being the most prevalent in the oropharynx. The secondary presence of Escherichia coli and Enterobacter aerogenes is reported in both sites. This result likely explains the high frequency of Klebsiella pneumoniae and Escherichia coli reported by Tadesse et al. [56] in clinical samples from Ethiopian patients with suspected infections, including both inpatients and outpatients. A study conducted in Tanzania reveals that Escherichia coli and Klebsiella pneumoniae are the most prevalent species of enterobacteria in clinical samples. Wilson and Gaido indicate that Escherichia coli is the most frequent cause of infections. Similar findings were also reported in Bahrain [57].
Regarding the resistance phenotype, the review made it possible to establish that amoxicillin was the least active antibiotic for oral enterobacteria, followed by ampicillin. On the other hand, beta-lactams associated with suicide inhibitors performed better. This pattern contrasts with the report by Goncalves et al. [30], who isolated strains of enterobacteria from subgingival niches in patients with chronic periodontitis that were susceptible to cephalosporins, aztreonam, carbapenems, aminoglycosides, and fluoroquinolones. These data suggest that the antimicrobial response profile among oral enterobacterial strains possibly varies from one niche to another. Therefore, from the analysis of antimicrobial activity against oropharyngeal enterobacteria, it was found that ampicillin was the least active antibiotic for all species isolated, followed by cephalosporins. The groups of antibiotics that demonstrated the best performance against species such as Escherichia coli, Klebsiella pneumoniae, Klebsiella aerogenes, Enterobacter cloacae, Raoultella planticola, Rahnella, Serratia marcescens, and Morganella morganii were carbapenems (imipenem and meropenem), aminoglycosides, sulfamethoxazole, and beta-lactams associated with suicide inhibitors. Based on the study by Le et al., 100% of the Escherichia coli isolates from the oropharynx were multidrug-resistant, given the carriage of genes encoding extended-spectrum β-lactamases such as the CTX-M9 group. Against these isolates, the only drugs that demonstrated efficient activity were carbapenems (imipenem and meropenem) [36]. The patients from whom these multidrug-resistant strains were obtained were elderly individuals in long-stay nursing homes. In fact, many studies associate the risk of carrying and spreading extended-spectrum β-lactamase-producing Enterobacteriaceae with operating theaters, prolonged hospital stays, inappropriate therapy, the use of indwelling catheters, and severe disease [58].
Age-related differences were found in the factors influencing oral or oropharyngeal colonization by enterobacteria. In elderly subjects, the main determinants are functional deterioration linked to terminal illness or severe disease, nasogastric tube feeding, bladder incontinence, and advanced chronic kidney disease. Comparisons with earlier evidence show concordance. In this context, the research by Samaranayake et al., published in 2004 [31], found that 71% of the participants were over 50 years old and were using antihypertensive, antidiabetic, psychiatric, cardiac, and antacid medications. These data suggest that in elderly subjects, systemic involvement is one of the main contributors to Enterobacteriaceae oral or oropharyngeal colonization. In addition, our consensus is comparable to that reported by Tada et al. [59], who reported an isolation rate of 16% of Enterobacter cloacae in dental plaque samples from people requiring systemic care. Alongside this, Tada and his group [59] reported an association between oral colonization by opportunistic pathogens and the incidence of pneumonia in hospitalized patients, finding Escherichia coli and Enterobacter cloacae in the dental plaque of eight patients.
In individuals under 65, the present review identified a range of factors contributing to oral or oropharyngeal colonization by Enterobacteriaceae, including the use of oral devices, parafunctional habits, smoking, periodontal disease, and poor oral hygiene. Likewise, Pathak et al. [32] found that intraoral devices are one of the primary factors governing the transition from the complex commensal community of the oral cavity to a source of pathogens, as they provide an additional site of adhesion and attachment in the form of biofilms, which act as a reservoir for pathogenic bacteria. In addition, the study by Pathak et al. [32] detected a wide variety of bacteria associated with orthodontic devices, including members of the Enterobacteriaceae family, anaerobic bacteria of the genera Lactobacillus and Streptococcus, Gram-positive bacilli of the genus Bacillus, and fungi of the genus Candida. In contrast, the study by Conti et al. [38] found no correlation between the presence of microorganisms and the use of oral prostheses. This result may be related to the small number of dental prosthesis users in the sample.
According to the study by Baydas et al. [29], parafunctional habits such as nail biting can lead to the autoinoculation of pathogens and the transmission of infections to other parts of the body, with a greater risk of fecal-oral transmission of enterobacteria in subjects who compulsively bite their nails compared to subjects without the habit. The study by Kamal and Bernard [28] supports the findings presented above. They observed that in children, the presence of harmful habits such as sucking fingers or biting nails, accompanied by poor hygiene, could result in debilitating systemic conditions. This is because habits associated with poor oral hygiene lead to a greater accumulation of dental plaque, which favors the colonization of numerous microorganisms, such as enterobacteria. Among them, Escherichia coli stands out, followed by species of Klebsiella, Proteus, and Enterobacter, whose spread through the oral cavity can lead to local and systemic infections [28]. On the other hand, Tischendorf et al. [60] reported in their study that chronic kidney disease is associated with essential pathogens capable of mediating disease in patients who carry them. In this study, 16.5% of infections were found in patients who were colonized with chronic kidney disease. Within this, men accounted for between 33 and 69%, with Klebsiella pneumoniae being one of the most frequently examined enterobacteria. For this reason, infections caused by chronic kidney disease have been associated with a considerable mortality rate of between 30 and 75%. Other factors such as prolonged hospital stays, a worse general state of health, comorbidities, and limited antimicrobial options to treat these infections also contribute to this high mortality rate [60].
Regarding the medical impact of asymptomatic colonization by this group of organisms, it has been proposed that enterobacteria, being opportunistic pathogens in patients with advanced chronic kidney disease, may lead to a potential increase in resistant infectious and inflammatory episodes in these patients. This contributes to the progression of kidney disease. According to Costa et al. [26], the accumulation of uraemic toxins in patients with advanced kidney failure and the consequent changes in the oral ecosystem can act as selection factors in the oral microbiome, leading to dysbiosis and the proliferation of pathobionts. Regarding the latter, studies in animal models have shown that the strains of Klebsiella pneumoniae with the capacity to colonize the intestinal tract efficiently and robustly spread from one site to another and from one host to another are those known as “hypervirulent”. This is a consequence of capsule expression [61] and multidrug resistance [43], both phenotypes linked to the carriage of accessory genomes in these pathobiont strains of Klebsiella pneumoniae [62]. This accessory genome, comprising plasmids and chromosomal gene loci, is responsible for dividing Klebsiella pneumoniae strains into opportunistic, hypervirulent, and multidrug-resistant strains. The former would be capable of causing disease in critically ill and immunocompromised patients. The second group would cause disease in healthy immunocompetent subjects. The third group would also behave as opportunists, causing infections that are difficult to treat. Therefore, it is this accessory genome that will determine whether a high aero-digestive colonization of Klebsiella pneumoniae remains asymptomatic or evolves into disease [62]. In addition, pathogenic strains of Escherichia coli, characterized by an adherent and invasive phenotype, have been linked to the onset and progression of inflammatory bowel disease due to their stimulatory factors that trigger the activation of the intestinal immune system [63]. To such an extent that a systematic review with meta-analysis demonstrated a substantial increase in the prevalence of these pathobiont strains of Escherichia coli in patients with inflammatory bowel disease compared to controls. The results of this study reported an odds ratio of 2.82 for ulcerative colitis and 3.27 for Crohn’s disease [64]. In this way, a pathogenic role was attributed to “adherent and invasive” strains of Escherichia coli in inflammatory bowel disease.
Regarding the dental impact of asymptomatic oral colonization by enterobacteria, various studies have shown that these bacteria are present in higher proportions in the periodontal pockets of patients with periodontitis compared to those of periodontally healthy individuals [30,40,41,42,65]. According to the observations of Vieira et al. [42], the subgingival colonization of enterobacteria is independent of the disease progression rate. On the other hand, in 2007, Botero et al. [65] demonstrated the presence of enterobacteria in subgingival niches in patients with aggressive periodontitis at a significantly higher frequency compared to subjects with chronic periodontitis and those with periodontally healthy teeth. In addition, substantial evidence placed Enterobacteriaceae as non-classical pathogens linked to the pathogenesis of periodontal disease, and capable of promoting its progression. This, after a systematic review, significantly higher levels and prevalence of enterobacteria in patients with this condition compared to periodontally healthy subjects. The presence of enterobacteria would be significant in aggressive forms of periodontitis [66]. In line with this, a 2014 review of periodontal microbiology in Latin America concluded that gram-negative enteric bacilli, similar to herpes viruses, could play a significant role in the pathogenesis of periodontal disease in the Latin American population. In this population, severe periodontal disease is prevalent, mainly in black and mestizo groups. This is linked to factors such as poverty and limited access to healthcare services, including periodontal care [67]. More analytical and longitudinal clinical observational studies are required, in addition to preclinical studies, to confirm the contribution of gram-negative enteric bacilli to the pathogenesis of rapidly progressing periodontal disease.
Recommendations
In light of the results of this literature review, oral health professionals must investigate, through thorough anamnesis, the determinants of upper aero-digestive colonization by enterobacteria. This aims to identify potential carriers of this bacterial group and reinforce in them the importance of measures such as oral hygiene, hand hygiene, and the controlled use of antibiotics. These are possible factors that can be intervened upon to prevent systemic infectious and inflammatory complications, despite this topic being rarely addressed in the dental field.
Conclusions
The presence of Enterobacteriaceae in unusual niches such as the mouth and oropharynx is significant. The genera Enterobacter, Klebsiella, and Escherichia are the dominant groups in both habitats. Strains with decreased susceptibility to beta-lactam antibiotics such as amoxicillin and ampicillin would mostly represent these genera.
Based on the evidence analyzed in this review, their presence would be favored and amplified by an inflammatory environment such as that provided by periodontal disease, in addition to other factors or determinants that vary according to age.
This asymptomatic carriage of enterobacteria in the upper aerodigestive tract is prevalent in children and adolescent subjects. Furthermore, it has systemic implications of an infectious and inflammatory nature, only in genetically predisposed hosts, those with immunological compromise, or those with pre-existing antibiotic-induced intestinal dysbiosis.
At the dental level, it is not yet possible to determine the role that enterobacteria would play in the pathogenesis of periodontal disease.

