Estudios originales
← vista completaPublicado el 6 de julio de 2022 | http://doi.org/10.5867/medwave.2022.06.002548
Asociación de biomarcadores y severidad de COVID-19: estudio transversal
Association of biomarkers and severity of COVID-19: A cross-sectional study
Abstract
Introduction COVID- 19 is a disease that has claimed the lives of many people. However, alterations in labo-ratory profiles in the city of Tacna have not been accurately established in association with its severity to support diagnosis and treatment.
Objective To determine biomarkers related to the severity of COVID- 19 in patients treated at the social security hospital in Tacna during 2020.
Methods We performed an observational, cross- sectional, and analytical study that included 308 patients with COVID- 19 from the social security hospital in Tacna, Peru, during the "first wave" of the pandemic (from July to August 2020). Immunological, hematological, arterial gas, hemostasis, and biochemical markers were collected. Patients were categorized into mild, moderate, and severe based on the clinical criteria found on clinical records. Correlation strength was per-formed according to Spearman’s Rho coefficient. The performance of the biomarkers associat-ed with severity was analyzed with the Receiver Operating Characteristic curve.
Results Regarding hematological markers there was a positive correlation with monocyte count (correla-tion coefficient: 0.841; area under the curve 97.0%; p < 0.05) and a negative correlation with lymphocyte count (correlation coefficient: -0.622; area under the curve 82.7%; p < 0.05). Regarding biochemical markers, arterial gases and hemostasis, no significant correlations were found. In immunological markers, we found positive correlation with ferritin (correlation coef-ficient: 0.805; area under the curve 94.0%; p < 0.05), and C- reactive protein (correlation coeffi-cient: 0.587; area under the curve 87.4%; p < 0.05).
Conclusions The biomarkers that can be considered as parameters associated with the severity of COVID- 19 are the absolute blood count of monocytes and serum ferritin concentration.
|
Main messages
|
Introduction
To the World Health Organization (WHO), COVID- 19 is the infectious disease caused by the SARS- CoV- 2 virus that first broke out in Wuhan (China) in late 2019. This infection generates various complications, including severe acute respiratory syndrome (SARS) [1].
From the pandemic onset until October 2021, more than 249 million people have been infected with this new virus, and 2.07% have died worldwide [2]. In addition, it has been observed that older people and those with comorbidities are more likely to develop a severe disease characterized by severe pneumonia or multiple organ failure, which are the leading causes of death in these patients [3]. The disease burden in Peru is high since more than 2 million confirmed cases of COVID- 19 and more than 200 000 deaths have been reported until October 2021 [2].
Several countries have widely described the clinical characteristics of COVID- 19, clearly defining its signs and symptoms. However, laboratory profiles have not been accurately established as biomarkers with prognostic value, nor have there been defined alterations that can help prevent patient complications [4].
However, several investigations have shown that biomarkers such as C- reactive protein, lactate dehydrogenase, T- troponin, ferritin, hematological parameters, and D- dimer are useful in clinical practice because they improve the categorization of patients according to severity [5]. Research by Sirvent J. et al. in Spain [6], Pujani M. et al. in India [7], and Galicia C. et al. in Mexico [8], have shown that these biomarkers are significantly altered in the multiple organ failure produced by COVID- 19, due to a sustained dysregulation of inflammatory response and organ hypoxia [9].
At the time of writing, there are more than 30 000 confirmed cases and 2 000 deaths due to COVID- 19 in Tacna, Peru [10]. Thus, there is an urgent need to standardize the laboratory bio-markers that account for Tacna’s epidemiology and identify patients who progress to severe stages [11].
This study aims to determine biomarkers related to the severity of COVID- 19 patients treated at the Tacna social security hospital. Also, it aims to establish criteria that allow risk stratification to perform timely interventions aimed at patients with a higher probability of developing severe complications.
Methods
The present observational, cross- sectional, analytical study was conducted at the social security hospital in Tacna, Peru, from June 1 to August 30, 2020. This work was approved by the Research Ethics Committee (CEI) of the Universidad Privada de Tacna, with Registration Code CEI- 001.
The research consisted of 308 patients with clinical findings of lower respiratory tract infection who tested positive for COVID- 19 through a rapid antigen test for detecting SARS- CoV- 2 or by the real- time reverse transcription- polymerase chain reaction assay (RT- PCR test).
Exclusion criteria included patients under 18 years of age and patients with a history of disorders that basally affect the count or dosage of the analytes tested as biomarkers of COVID- 19 severity (e.g., thrombocytopenic purpura, allergies, leukemia).
Data were collected from the laboratory management system, which considered the collection of serum, plasma, and whole blood samples from patients on admission. Ferritin, troponin I, and procalcitonin were tested using the electrochemiluminescence method on the COBAS E411 automated analyzer from Roche Diagnostics. Hematological biomarkers (absolute counts of leukocytes, eosinophils, basophils, neutrophils, lymphocytes, and platelets) were measured with the Sysmex XS- 1000i five- strain automated device from Roche Diagnostics. Biomarkers obtained from arterial gases (pH, partial oxygen and partial car-bon dioxide pressure, arterial oxygen saturation, fractional per-centage of inspired oxygen, and bicarbonate, sodium, potassium, chloride, ionized calcium, and lactate concentration) were measured with automated ABL800 FLEX from Radiometer. Coagulation biomarkers (prothrombin time, fibrinogen, and D- dimer) were measured with the Coatron A6 Plus automated equipment from TECO Medical Instruments. Biochemical biomarkers (lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, total creatine phosphokinase, myocardial creatine phosphokinase, glucose, urea, and creatinine) were estimated with the Bio System BA400 automated equipment, in addition to C- reactive protein, which is an immunological marker. The analytical methods were per-formed according to the manufacturer’s indications, considering internal and external quality controls.
Patients were categorized into mild, moderate, and severe based on the clinical criteria found on medical records. Mild disease was defined as patients with mild symptoms that showed no changes in pulmonary imaging. Moderate disease was defined as having one of the following symptoms: oxygen saturation at room air less than or equal to 93%, respiratory rate greater than or equal to 30 breaths per minute, alveolar partial pressure of oxygen/fractional percentage of inspired oxygen ratio less than 300 millimeters of mercury, and pulmonary infiltrates greater than 50% within 24 to 48 hours of symptom onset [12]. Critical disease was defined as patients presenting respiratory failure with mechanical ventilation and requiring follow- up in the intensive care unit.
For statistical analysis, the Kolmogorov- Smirnov test was applied to corroborate the normality of the sample. Correlation strength between variables was performed according to Spearman’s Rho coefficient. The hematological, biochemical, immunological, arterial gas, and hemostasis biomarker analytes were expressed according to mild, moderate, and severe categories in mean plus or minus one standard deviation (SD). Differences between means of biomarker values were analyzed using the Wilcoxon signed- rank test for independent samples.
The biomarkers associated with COVID- 19 severity were per-formed with the Receiver Operating Characteristic (ROC) curve and the area under the curve (AUC). The performance of the aforementioned statistical tests was assessed using SPSS Statistics Data software version 25, with p < 0.05 considered statistically significant.
The information was collected between July and August 2020, when the "peak" of the pandemic wave hit the city of Tacna in Peru, which is why most of the population has a moderate to severe stage of COVID- 19.
The shortage of reagents and the lack of a standardized laboratory testing protocol for COVID- 19 were limiting factors to studying a larger population.
Results
We evaluated 308 participants with a diagnosis of COVID- 19. We identified 36 laboratory analytes in the following areas: hematology, biochemistry, immunology, hemostasis, arterial gases, and electrolytes.
Of the total population, 69% were male, with a mean age of 61.2 ± 13.7 years, and 37% showed some comorbidity.
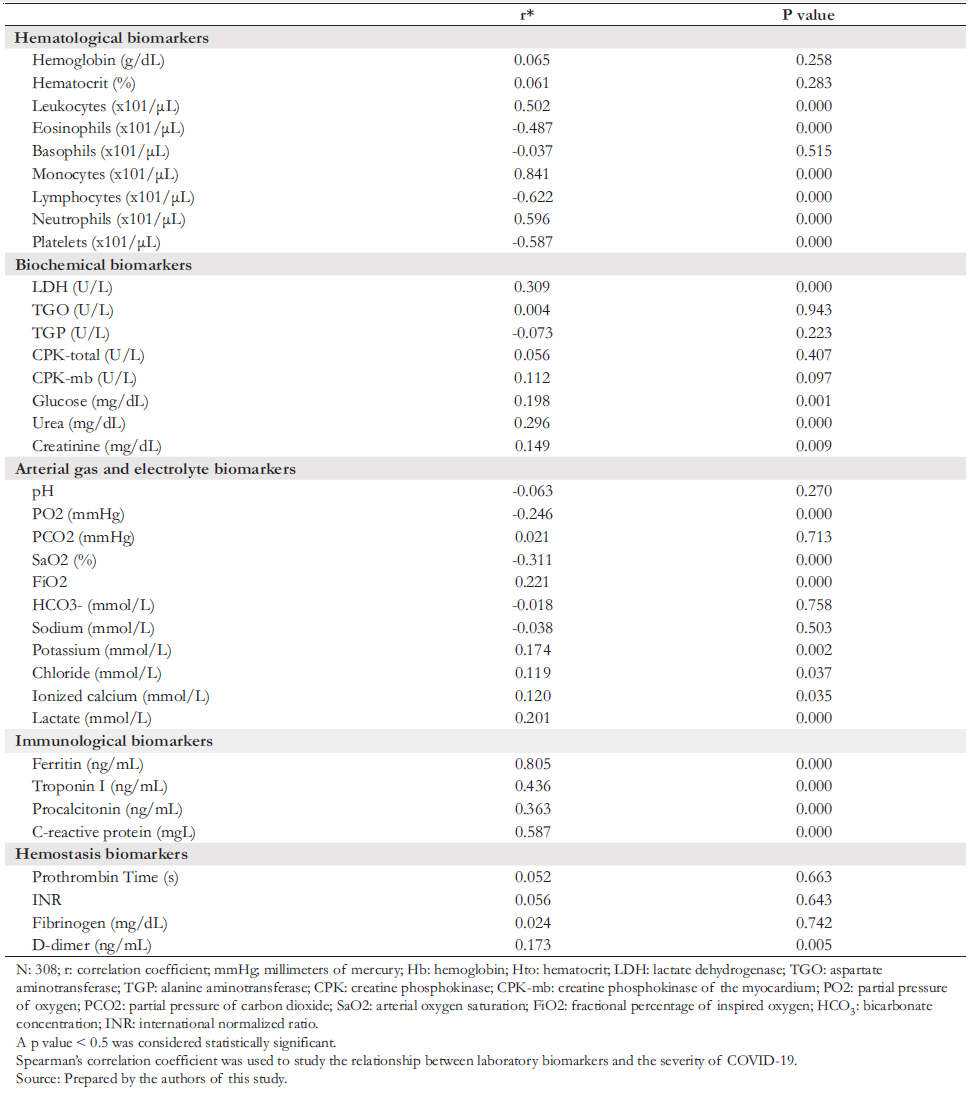
Table 1 shows that out of 308 eligible patients with COVID- 19, the mean age was 61.2 years (confidence interval: 59.6 to 62.7 years), the female population represented 31% (mean age: 64.5 years), and the male population represented 69% (mean age: 59.7 years). Thirty- six percent of the study population reported some comorbidity, the most frequent being diabetes (17%) and arterial hypertension (8%). When correlating the degree of severity of COVID- 19 with laboratory analytes, we observed positive and negative correlations with various parameters.
 Full size
Full size Concerning the positive correlation, we found that this was:
a) Of high degree with absolute monocyte count and serum fer-ritin concentration.
b) Of moderate degree with the absolute leukocyte count, neutrophils, troponin I, and C- reactive protein.
c) Of low degree with lactate dehydrogenase, urea, procalcitonin, and lactate.
d) Of very low degree with blood potassium, chloride, ionized calcium, D- dimer, glucose, and creatinine.
Likewise, the absolute lymphocyte count was highly and negatively correlated with COVID- 19 severity. Moreover, the absolute eosinophil and platelet count (moderate degree) and partial pressure of oxygen and arterial oxygen saturation (low degree) negatively correlated with COVID- 19 severity.
Absolute monocyte count and serum ferritin concentration showed the strongest correlation with the degree of severity of COVID- 19.
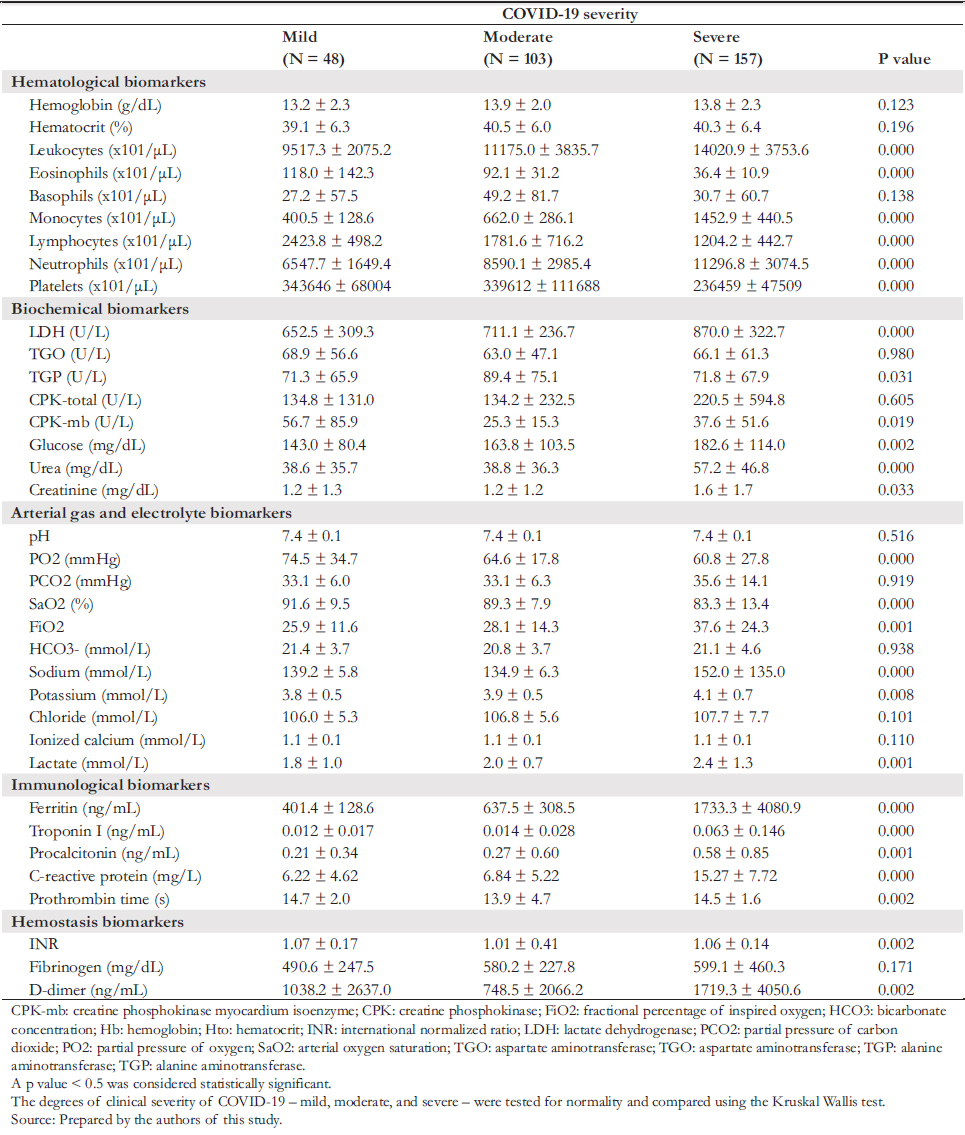
The degrees of clinical severity of mild, moderate, and severe COVID- 19 were tested for normality and compared using the Kruskal Wallis test.
Table 2 shows a statistically significant difference (p < 0.05), where the absolute count of leukocytes, monocytes, neutrophils, serum concentration of lactate dehydrogenase, alanine aminotransferase, creatine kinase myocardium isoenzyme, glucose, urea, creatinine, percentage of inspired oxygen, sodium, potassium, lactate, ferritin, troponin I, procalcitonin and C- reactive protein, and D- dimer were higher in patients with severe COVID- 19 than in those with mild and moderate COVID- 19. Meanwhile, the absolute count of eosinophils, lymphocytes, platelets, and partial pressure of oxygen and arte-rial oxygen saturation was lower in patients with severe COVID- 19 than in those with mild and moderate COVID- 19.
 Full size
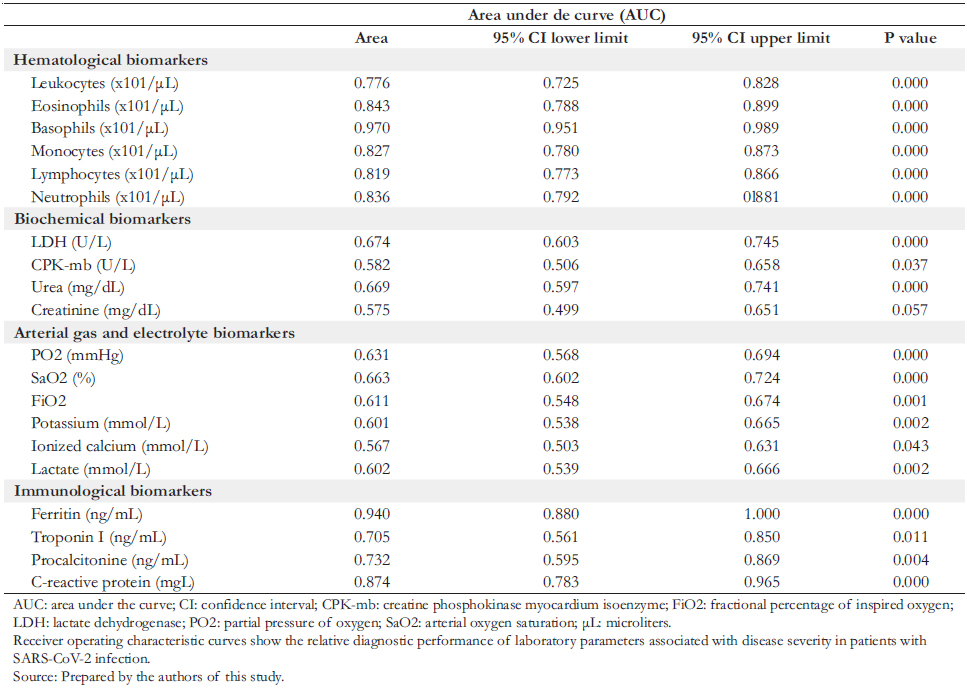
Full size ROC curves (shown in Table 3 and Figure 1) were con-structed to evaluate the association of the severity of COVID- 19 patients at admission with laboratory biomarkers. The results showed that the area under the curve (AUC) with the highest performance in hematological biomarkers were monocytes (AUC: 97%) and eosinophils (AUC: 84.3%); in biochemical biomarkers, were lactate dehydrogenase (AUC: 67.4%) and urea (AUC: 66.9%); in arterial gas biomarkers were arterial oxygen saturation (AUC: 66.3%) and partial oxy-gen saturation (AUC: 63.1%); while in immunological bio-markers were ferritin (AUC: 94%) and C- reactive protein (AUC: 87.4%).
The laboratory analytes that showed the highest correlation with severe disease in COVID- 19 patients were absolute monocyte blood count, serum ferritin concentration, and C- reactive protein.
 Full size
Full size 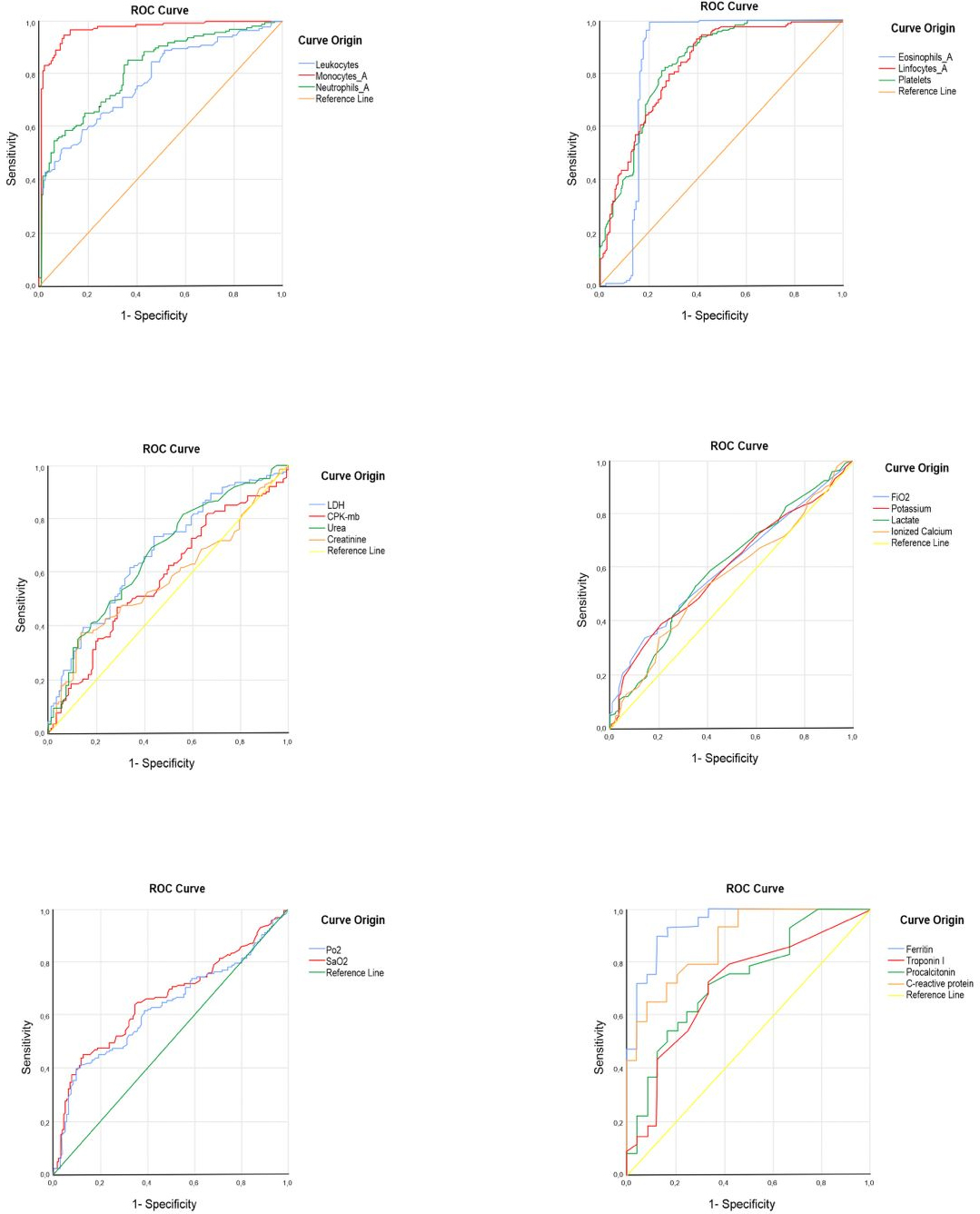 Full size
Full size Discussion
This study showed a high percentage (36%) of comorbidity among eligible participants. These results resemble those found in a study in Peru in 2020, where existing comorbidities were present in almost 40% of patients [13]. We found a positive correlation between the severity of COVID- 19 and the absolute monocyte count and serum ferritin concentration. These results align with Lippi G. et al. and Zeng et al. findings [4],[9], who found hematological counts and serum ferritin as markers of disease severity progression. However, it should be taken into account that these studies were performed in other settings and that our work presented certain methodological limitations, including incomplete data on clinical records, which negatively impacted the sample size of this study.
We found that a severe COVID- 19 disease was significantly associated (p < 0.05) with absolute leukocyte, monocytes, neutrophils count, and serum concentration of lactate dehydrogenase, alanine aminotransferase, creatine kinase myocardium isoenzyme, glucose, urea, creatinine, ferritin, troponin I, procalcitonin, C- reactive protein, and plasma D- dimer concentration. Regarding biomarker performance, absolute blood counts, ferritin, and C- reactive protein were strongly associated with severe disease.
Regarding hematological biomarkers, we found a positive correlation between the total leukocyte, monocyte, and neutrophil counts and the severity of COVID- 19. These results agree with Michael B. et al. study [14], where a positive correlation was found in both leukocyte and neutrophil counts. On the other hand, a meta- analysis performed by Ghahramani S. et al. [15] found a negative correlation with lymphocyte, eosinophil, and platelet counts, which also aligns with our results.
Several studies conducted in different settings, such as Mardani R. et al. in Iran, Dong X. et al. in China, Bennouar S. et al. in Algeria, and Aloisio E. et al. in Italy [16],[17],[18],[19], indicate that there is a significant correlation between lactate dehydrogenase and the degree of severity of patients diagnosed with COVID- 19. These results support our findings. In addition to these bio-markers, we found a significant correlation with glucose, creatinine, and urea.
Concerning arterial gas and electrolyte biomarkers, we found a low positive correlation of oxygen partial pressure, fractional percentage of inspired oxygen, potassium, chloride, ionized calcium, and lactate with disease severity. Moreover, we found a negative correlation between arterial oxygen saturation and dis-ease severity.
Gutiérrez J. et al. [20] found that troponin I and procalcitonin correlate with the severity of patients with COVID- 19. On the other hand, Huang I. et al. [21] found that C- reactive protein, procalcitonin, and ferritin also correlate with disease severity. These findings align with our results.
Regarding coagulation biomarkers, we found no correlation between the degree of disease severity and the prothrombin time, international normalized ratio, and fibrinogen tests. In contrast, the D- dimer test showed a positive correlation. Despite epidemiological differences with our work, Long H. et al. [22], in their research conducted at the Tianyou Hospital of Wuhan University of Science and Technology, reported similar results concerning the D- dimer. However, they also found a positive correlation between the measurement of fibrinogen and the severity of COVID- 19.
In our setting, we found results shared with other studies on the differences in hematological biomarkers according to the severity stratification of patients with COVID- 19. Gallo B. et al. [23], Tan L. et al. [24], and Henry B. et al. [25] reported decreased lymphocyte counts in patients diagnosed with severe COVID- 19. Lippi G [26]. and Tjendra Y. et al. [27] found an association between low platelet count and the severe severity of COVID- 19. On the other hand, Tuta E. et al. [28] and Dinevari M [29]. found low hemoglobin levels in severe cases of COVID- 19, agreeing with our findings on lymphocytes and platelets, but not with hemoglobin levels. The differences in the results may be due to the nature of the studies or the type of population.
Moreover, we found that most of the biochemical and immunological tests increased with the severity of the disease, except for aspartate aminotransferase and total creatine phosphokinase. These results align with Gutiérrez et al. [20] and Ocampo C et al. [30] in their work on biomarkers.
In all, we found that hematological and immunological examinations had the highest correlation with disease severity. The parameters with the best performance were eosinophil count, monocytes, lymphocytes, neutrophils, platelets, C- reactive protein, and ferritin. These results share similarities with research conducted in other settings, such as those of Fang L. et al. [31], Gutiérrez C. et al. [20], and Ocampo C. et al. [30].
It should be emphasized that it is of utmost importance to establish risk and diagnostic profiles involving the measurement of biomarkers for the management or prognosis of patients with COVID- 19.
Limitations
Finally, it should be mentioned that our study has certain limitations. Among them is the pandemic setting in which the data collection was performed, which limited the available information. Moreover, the management of COVID- 19 patients varied continuously as the scientific knowledge and guidelines changed over time. On the other hand, the sample size was relatively small for reasons unrelated to the study, limiting the extrapolation of results to other regions in Peru.
Conclusions
With the so- called "wave peak", the COVID- 19 pandemic presented enormous challenges to the healthcare system. Amidst critical scenarios, it is necessary to ensure rational use of medical resources to treat patients with severe diseases effectively. However, management requires early diagnostic tools to reduce mortality in the affected population. In the present work, we investigated the strength of correlation between laboratory bio-markers and the degree of severity of COVID- 19 disease. We found that the absolute monocyte count and serum ferritin concentration had the highest correlation with COVID- 19 severity. These findings establish these laboratory biomarkers as valuable parameters to infer the prognosis of hospitalized COVID- 19 patients.
This study proposes a mathematical approach that integrates monocytes and ferritin as an index of inflammation and, possibly, as a predictor of the dysregulation of the inflammatory response produced by COVID- 19.
Considering that the data was collected at the "peak" of the first wave of the pandemic in the city of Tacna, further research with larger samples is recommended to confirm our findings.
Implications
With the results obtained in this work, it may be possible:
- To standardize critical values of COVID- 19 biomarkers available in Tacna, Peru, according to population charac-teristics and logistic feasibility.
- To analyze laboratory markers that signal cardiac, muscular, renal, and thrombotic complications – significantly elevated in multiple organ failure – and are associated with COVID- 19 severity.
- To encourage further studies to develop clinical diag-nostic tools that integrate, through a mathematical index, laboratory analytes that show high statistical correlation with the severity of COVID- 19 to allow the diagnosis and timely treatment of the severe or critical disease states.

