Resúmenes Epistemonikos
← vista completaPublicado el 3 de diciembre de 2015 | http://doi.org/10.5867/medwave.2015.6326
¿Es útil el inicio temprano de la terapia antirretroviral en adultos VIH(+) sin coinfecciones?
Is early antiretroviral therapy initiation useful in HIV(+) adults without co-infections?
Abstract
HIV infection is a worldwide epidemic. Antiretroviral therapy has dramatically changed the outcome of the disease but there is still controversy about the best time to initiate it, especially in patients with CD4 counts over 350 cells/µL. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified two systematic reviews including four pertinent randomized controlled trials overall. We concluded early initiation of antiretroviral therapy probably reduces mortality, risk of opportunistic infections and tuberculosis, but increases the risk of important adverse effects.
Problem
Human immunodeficiency virus (HIV) infection is a worldwide epidemic. The use of antiretroviral therapy has changed the outcome of the disease, clearly and consistently reducing mortality. There is wide agreement about the benefits of starting antiretroviral therapy in patients at B or C stage, CD4 count below 350, during pregnancy or lactation, in patients co-infected with hepatitis C virus, hepatitis B virus or tuberculosis, HCV, and in patients over 50 years of age. However, there is no consensus about the effects of early initiation of antiretroviral therapy in patients with CD4 counts over 350.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found two systematic reviews [1],[2], that consider 23 primary studies reported in 25 references [3],[4],[5],[6], [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18], [19],[20],[21],[22],[23],[24],[25],[26],[27], including four randomized controlled trials reported in six references [6],[11],[12],[13],[16],[20]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
Two studies were conducted in multiple countries [6],[11], one in Haiti [13] and one in the USA [16]. All of the studies included patients over 18 years of age, except one study that included younger patients (over 13 years) [11]. All studies included HIV infected adults, naïve to treatment and willing to be treated. One study included patients with CD4 counts equal or greater than 350 [11], one with CD4 counts between 200 and 350 [13], one with CD4 counts between 350 and 500 [6] and other with CD4 counts equal or greater than 350 [16]. Average of CD4 counts across studies was 337. All studies excluded pregnant or nursing women, patients in advanced stage of disease (AIDS) or with previous use of antiretroviral therapy. Two studies included normal laboratory parameters among their inclusion criteria [6],[16]. |
|
What types of interventions were included |
The interventions were:
|
|
What types of outcomes |
The following outcomes were measured:
|
Summary of findings
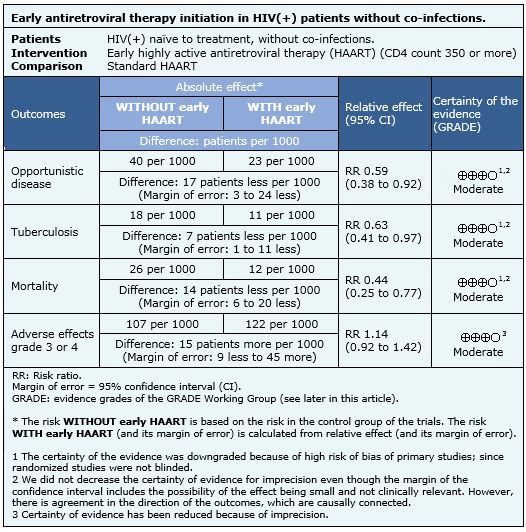
The information on the effects of early initiation of antiretroviral therapy in HIV infected patients with CD4 counts over 350 is based on four randomized controlled trials [6],[11],[13],[16], that include 4,686 patients. Two studies [6],[11], measured the outcome opportunistic infections, three [6],[11],[13] the outcome tuberculosis, three [6],[11],[13] measured mortality and two studies [6],[13] measured grade 3-4 adverse events.
- Early initiation of antiretroviral therapy probably reduces the incidence of opportunistic diseases. The certainty of the evidence is moderate.
- Early initiation of antiretroviral therapy probably reduces the incidence of tuberculosis. The certainty of the evidence is moderate.
- Early initiation of antiretroviral therapy probably reduces mortality in HIV infected patients. The certainty of the evidence is moderate.
- Early initiation of antiretroviral therapy probably increases the risk of grade 3-4 adverse events. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
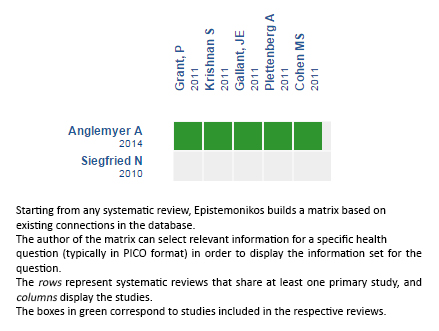
Follow the link to access the interactive version: Early initiation of antiretroviral therapy in HIV-infected adults without coinfection
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

