Epistemonikos summaries
← vista completaPublished on December 21, 2016 | http://doi.org/10.5867/medwave.2016.6801
What is the role of recombinant activated protein C in the management of sepsis?
¿Cuál es el rol de la proteína C activada en el tratamiento de la sepsis?
Abstract
During an episode of sepsis, systemic inflammatory response phenomenon triggers a series of procoagulant mechanisms. It has been suggested that the use of activated protein C could play a role in the management of this pathology, but there is no consensus. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified seven systematic reviews covering 35 primary studies addressing the question of this article, including six randomized trials. We extracted data, combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded activated protein C in sepsis probably does not decrease the mortality rate and increases the rate of hemorrhagic events.
Problem
Sepsis continues leading the causes of morbidity and mortality in intensive care units. Its incidence has been increasing, with greater complications and with more resistant infectious agents. While there has been some tendency to decreased mortality through some interventions, effective therapeutic tools remain limited.
Human protein C is a vitamin K dependent glycoprotein, structurally similar to other proteins that affect blood clotting, such as prothrombin, Factor VII, Factor IX and Factor X. It is thought that it would play an important role by regulating the anticoagulation, inflammation, cell death and maintaining the permeability of the walls of blood vessels.
The pharmaceutical company that produced this drug withdrew it from the market in 2011.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found seven systematic reviews [1],[2],[3],[4],[5],[6],[7], including 35 primary studies [8],[9],[10],[11], This table and the summary in general are based on the latter. |
|
What types of patients were included |
Regarding the types of patients, it should be noted that the six trials were carried out after 1991, the year that the first consensus definition for sepsis and septic shock was performed, so that the definition and graduation of the severity of sepsis is homogenous between trials. |
|
What types of interventions were included |
In four of six trials, the type of activated protein C used was drotrecogin alpha-activated [8],[11],[12],[13] and in two was activated human C protein (rhAPC) [9],[10]. The route of administration was intravenous in all trials. Regarding the dose, five used a standard dose of 24 μg/kg/hour for a total of 96 hours [8],[10],[11],[12],[13]. One trial used variable doses and different time than 96 hours [9]. All trials compared against placebo or standard treatment. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
Both trials evaluated the use of tetrahydrocannabinol capsules administered orally. In one trial, the dose was 5 mg, 7.5 mg or 10 mg once [9], and in the other trials, the dose was not specified [8].
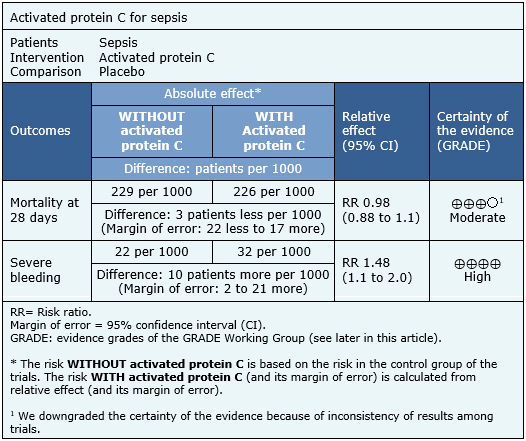
Summary of findings
The information on the effects of activated protein C on sepsis is based on six randomized trials including 6,781 patients. All trials measured the outcome mortality at 28 days and severe bleeding.
The summary of findings is as follows:
- The use of activated protein C in patients with sepsis probably does not decrease the mortality rate. The certainty of the evidence is moderate.
- The use of activated protein C in patients with sepsis increases the rate of severe bleeding during hospitalization. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
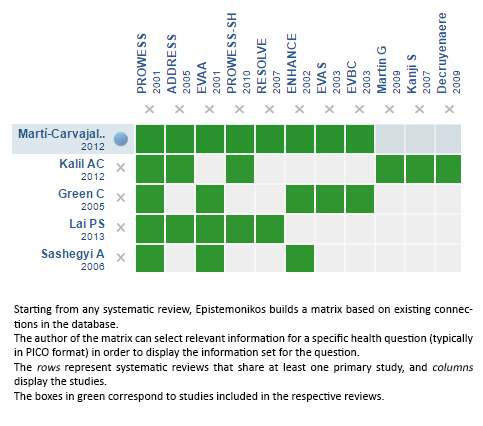
Follow the link to access the interactive version: Recombinant activated protein C for the treatment of sepsis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

